Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Monika Šabić Runjavec.
Hydrogen technology has great potential as a source of clean energy. The production of green hydrogen is a desirable and beneficial way to contribute to the decarbonization of the energy sector. In response to the demand for environmentally friendly and economically feasible approaches, biohydrogen production from waste materials has attracted interest. Waste materials from industrial or municipal production can be used as low-cost substrates for biohydrogen production through microbial degradation.
- biohydrogen
- waste stream
- industrial wastewater
1. Biological Processes for Hydrogen Production
The various biological processes used for the production of hydrogen can be classified into photosynthetic processes, fermentative processes and processes with microbial electrolysis cells (MECs). Photosynthetic processes (direct and indirect biophotolysis) are biological processes in which solar energy is used to drive water-splitting photosynthesis and to generate hydrogen from the energy-rich electrons produced in the process by photosynthetic microorganisms (green algae, cyanobacteria) [2][1]. The MEC process is a biological hydrogen production process in which a hydrogen evolution reaction is catalyzed by electroactive bacteria in the presence of anaerobic conditions [27][2]. Anaerobic fermentation is one of the most commonly used processes for biohydrogen production from waste substrates [16][3]. Depending on the types of microorganisms used and whether they require light to maintain their life cycle, fermentation processes can be divided into photo-fermentation, dark fermentation and processes integrating photo- and dark fermentation [28][4].2. Microbiology of Biohydrogen-Producing Systems
The biological processes of biohydrogen production are directly influenced by the process conditions, which must meet the requirements for microbial growth. These processes are catalyzed by microorganisms under optimal environmental conditions. The characteristics of these microorganisms differ greatly depending on the substrate and the process conditions [15][5]. When substrates with high organic content are used for biohydrogen production, such as industrial wastewater and waste activated sludge, the leading biological method is the process of dark fermentation, which is discussed in this section. In order to realize the two main advantages of waste management and energy generation—or the principle of “waste-to-biohydrogen”—organic waste is preferred as the substrate for hydrogen production via the fermentation process. Real waste samples typically contain complex compounds, such as polysaccharides, proteins and lipids. As polysaccharides can be more easily converted to hydrogen, a high hydrogen yield can be obtained, while proteins and lipids have lower energy conversion efficiency. However, they are essential for microbial growth [13][6]. The most favorable condition for biohydrogen production from different types of organic waste is a high concentration of soluble substrate. Mesophilic conditions are also preferred because they require lower temperatures and lower energy consumption compared to thermophilic conditions. An acidic pH, which can be maintained without the addition of chemicals, increases the attractiveness and environmental sustainability of this process. In addition, the short residence time means a smaller reactor volume, which helps to reduce the capital and operating costs of the process [29][7]. The microorganisms present in biohydrogen-producing systems can be defined as hydrogen producers or non-hydrogen producers (Figure 1). In terms of their hydrogen-producing metabolism, hydrogen producers can be classified as photosynthetic microorganisms or fermentative microorganisms (photo-fermentation—e.g., Rhodobacter, Chromatium; and dark fermentation—e.g., Clostridium, Enterobacter, Citrobacter). Hydrogen-producing systems are heterogeneous and interacting ecosystems. In addition to the dependence of the biohydrogen yield on the hydrogen producers, other microbial groups also contribute to the essential functionality of the ecosystem. Microorganisms that are not able to produce hydrogen are categorized as non-hydrogen producers, with the three subgroups of hydrogen consumers, substrate contenders and beneficial bacteria. The undesirable microbial group of hydrogen consumers can reduce the efficiency of biohydrogen production systems due to their consumption of the produced hydrogen and their forming of methane or acetate. Substrate contenders compete with hydrogen producers for the substrate and in this way negatively influence the biohydrogen yield [13][6].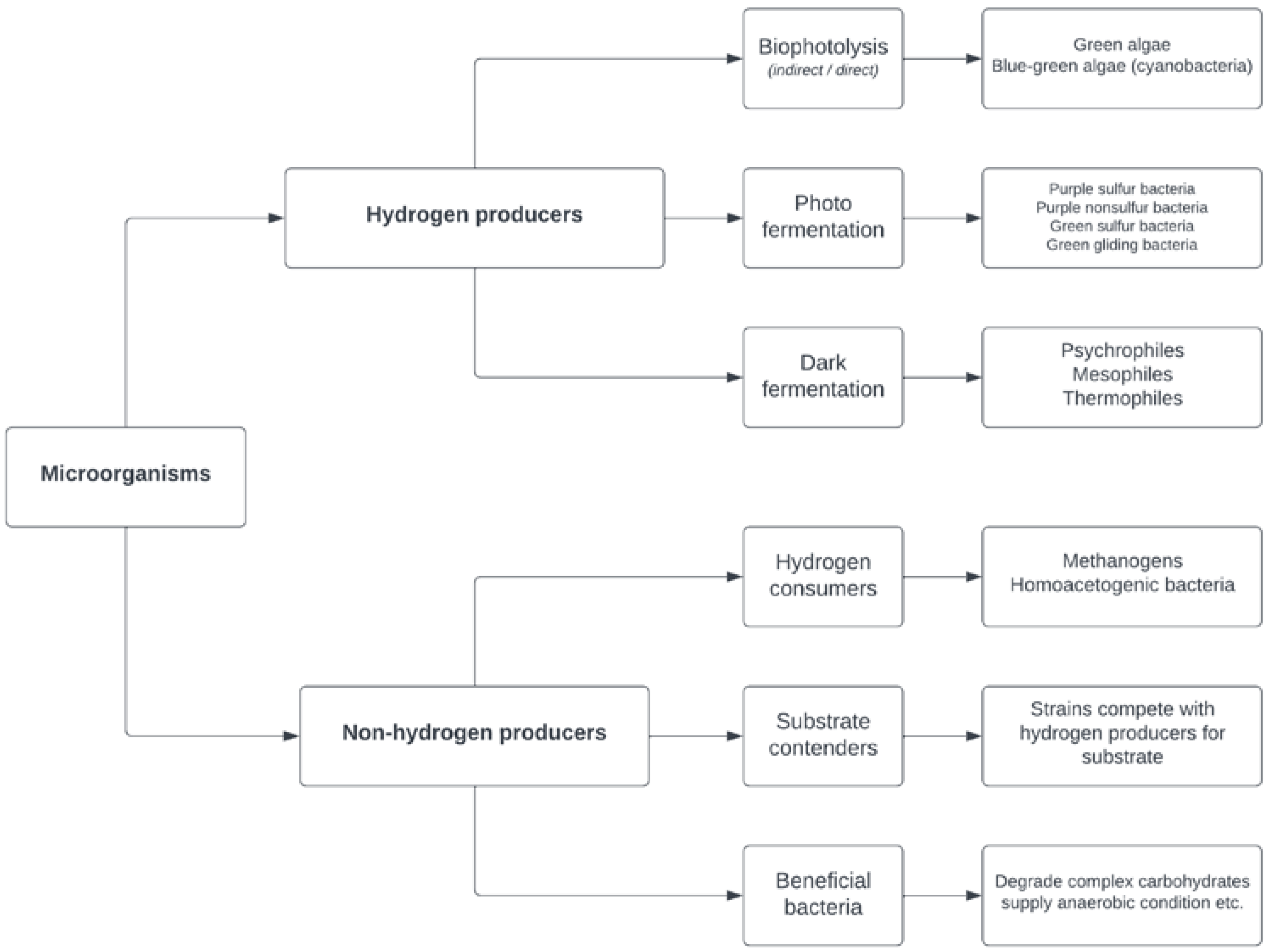
Figure 1.
Microbial groups present in hydrogen-producing systems.
3. Role of Microorganisms in Dark Fermentation Process
The dark fermentation process is the most widely studied fermentation process for the utilization of waste materials. In this process, hydrogen-producing microorganisms can use organic fractions of waste materials as a source of carbon and produce biohydrogen without light and oxygen. The production of biohydrogen through dark fermentation has several advantages, such as a high production rate, the ability to efficiently use a variety of organic waste substrates, sustainability [32,33][10][11] and no requirement for light energy [34][12]. The disadvantages of the dark fermentative process include the accumulation of hydrogen in the fermentative system, which can lead to inhibition of bacterial metabolism [35][13], and the relative sensitivity of the hydrogenate enzyme in dark-fermentative bacteria to oxygen, which can lead to lower hydrogen yields [36][14]. Microorganisms involved in the dark fermentation process belong to the groups of facultative anaerobic bacteria or obligate anaerobic bacteria. In dark fermentation, several biochemical processes occur in parallel with those of anaerobic digestion. Different microbial strains act synergistically to contribute to the degradation of organic compounds. The process of anaerobic digestion can be divided into four phases: hydrolysis, acidogenesis, acetogenesis and methanogenesis, with the last phase of methanogenesis being excluded in dark fermentation (Figure 2).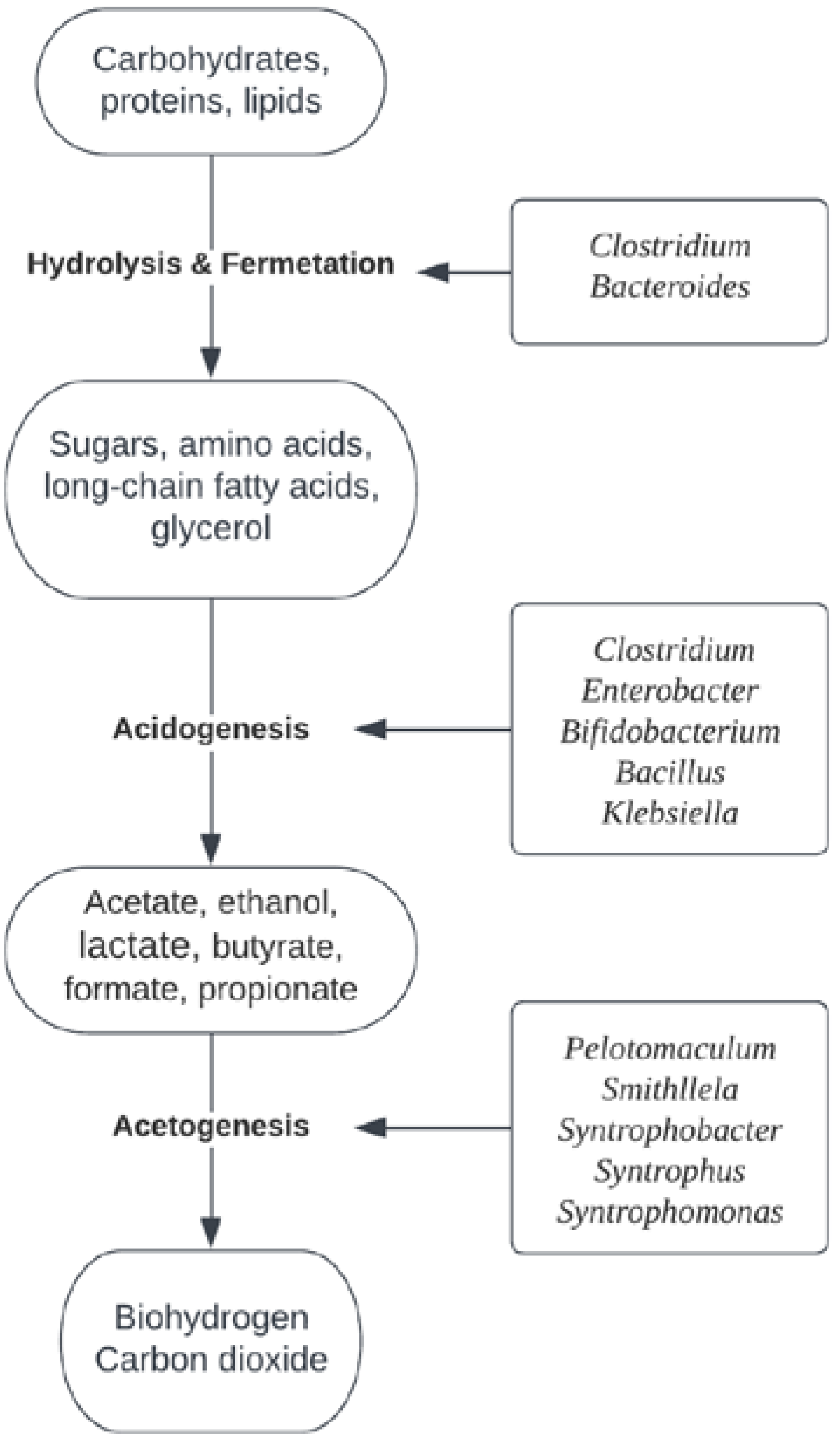
Figure 2.
The biochemical pathway and dominant microbial genera in the dark fermentation process for organic complex compounds.
C
6
H
12
O
6
+ 2H
2
O → 2CH
3
COOH + 4H
2
+ 2CO
2
C6H12O6 → CH3CH2CH2COOH + 2H2 + 2CO2
C
6
H
12
O
6
→ CH
3
COOH + CH
3
CH
2
COOH + H
2
+ CO
2
References
- Hallenbeck, P.C.; Zampol Lazaro, C.; Sagir, E. Biology and Physiology of Photobiological Hydrogen Production. In Microalgal Hydrogen Production: Achievements and Perspectives; Seibert, M., Torzillo, G., Eds.; Royal Society of Chemistry: London, UK, 2018; pp. 1–30.
- Jensen, L.S.; Kaul, C.; Juncker, N.B.; Thomsen, M.H.; Chaturvedi, T. Biohydrogen Biohydrogen Production in Microbial Electrolysis Cells Utilizing Organic Residue Feedstock: A Review. Energies 2022, 15, 8396.
- Kanwal, F.; Torriero, A.A.J. Biohydrogen—A Green Fuel for Sustainable Energy Solutions. Energies 2022, 15, 7783.
- Cheng, D.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Deng, L.; Chen, Z.; Ye, Y.; Bui, X.T.; Hoang, N.B. Advanced strategies for enhancing dark fermentative biohydrogen production from biowaste towards sustainable environment. Bioresour. Technol. 2022, 351, 127045.
- Bharathiraja, B.; Sudharsanaa, T.; Bharghavi, A.; Jayamuthunagai, J.; Praveenkumar, R. Biohydrogen and Biogas—An overview on feedstocks and enhancement process. Fuel 2016, 185, 810–828.
- Wang, J.; Yin, Y. Progress in microbiology for fermentative hydrogen production from organic wastes. Crit. Rev. Environ. Sci. Technol. 2019, 49, 825–865.
- Moussa, R.N.; Moussa, N.; Dionisi, D. Hydrogen Production from Biomass and Organic Waste Using Dark Fermentation: An Analysis of Literature Data on the Effect of Operating Parameters on Process Performance. Processes 2022, 10, 156.
- Cabrol, L.; Marone, A.; Tapia-Venegas, E.; Steyer, J.-P.; Ruiz-Filippi, G.; Trably, E. Microbial ecology of fermentative hydrogen producing bioprocesses: Useful insights for driving the ecosystem function. FEMS Microbiol. Rev. 2017, 41, 158–181.
- Chou, C.H.; Han, C.L.; Chang, J.J.; Lay, J.-J. Co-culture of Clostridium beijerinckii L9, Clostridium butyricum M1 and Bacillus thermoamylovorans B5 for converting yeast waste into hydrogen. Int. J. Hydrog. Energy 2011, 36, 13972–13983.
- Fatima, A.; Basak, B.; Ganguly, A.; Chatterjee, P.K.; Dey, A. Biohydrogen Production through Dark Fermentation of Food Wastes by Anaerobic Digester Sludge Mixed Microbial Consortium. In Recent Developments in Waste Management; Kalamdhad, A., Ed.; Springer: Singapore, 2020; pp. 57–70.
- Słupek, E.; Kucharska, K.; Gębicki, J. Alternative methods for dark fermentation course analysis. SN Appl. Sci. 2019, 1, 469.
- Rambabu, K.; Bharath, G.; Thanigaivelan, A.; Das, D.B.; Show, P.L.; Banat, F. Augmented biohydrogen production from rice mill wastewater through nano-metal oxides assisted dark fermentation. Bioresour. Technol. 2021, 319, 124243.
- Singh, R.; White, D.; Demirel, Y.; Kelly, R.; Noll, K.; Blum, P. Uncoupling fermentative synthesis of molecular hydrogen from biomass formation in thermotoga maritima. Appl. Environ. Microbiol. 2018, 84, e00998-18.
- Hosseinzadeh, A.; Zhou, J.L.; Li, X.; Afsari, M.; Altaee, A. Techno-economic and environmental impact assessment of hydrogen production processes using bio-waste as renewable energy resource. Renew. Sustain. Energy Rev. 2022, 156, 111991.
- Dzulkarnain, E.L.N.; Audu, J.O.; Dagang, W.R.Z.W.; Abdul-Wahab, M.F. Microbiomes of biohydrogen production from dark fermentation of industrial wastes: Current trends, advanced tools and future outlook. Bioresour. Bioprocess 2022, 9, 16.
- Li, W.; Guo, J.; Cheng, H.; Wang, W.; Dong, R. Two-phase anaerobic digestion of municipal solid wastes enhanced by hydrothermal pretreatment: Viability, performance and microbial community evaluation. Appl. Energy 2017, 189, 613–622.
- Saravanan, A.; Kumar, P.S.; Khoo, K.S.; Show, P.-L.; Carolin, C.F.; Jackulin, C.F.; Jeevanntham, S.; Karishma, S.; Show, K.-Y.; Lee, D.-J.; et al. Biohydrogen from organic wastes as a clean and environment-friendly energy source: Production pathways, feedstock types, and future prospects. Biores. Technol. 2021, 342, 126021.
- Saravanan, A.; Senthil Kumar, P.; Aron, N.S.M.; Jeevanantham, S.; Karishma, S.; Yaashikaa, P.R.; Chew, K.W.; Show, P.L. A review on bioconversion processes for hydrogen production from agro-industrial residues. Int. J. Hydrog. Energy 2022, 47, 37302–37320.
- Patel, S.K.S.; Das, D.; Kim, S.C.; Cho, B.-K.; Kalia, V.C.; Lee, J.K. Integrating strategies for sustainable conversion of waste biomass into dark-fermentative hydrogen and value-added products. Renew. Sustain. Energy Rev. 2021, 150, 111491.
- Islam, A.K.M.K.; Dunlop, P.S.M.; Hewitt, N.J.; Lenihan, R.; Brandoni, C. Bio-Hydrogen Production from Wastewater: A Comparative Study of Low Energy Intensive Production Processes. Clean Technol. 2021, 3, 156–182.
- Ergal, I.; Gräf, O.; Hasibar, B.; Steiner, M.; Vukotić, S.; Bochmann, G.; Fuchs, W.; Rittmann, S.K.M.R. Biohydrogen production beyond the Thauer limit by precision design of artificial microbial consortia. Commun. Biol. 2020, 3, 443.
- Ergal, I.; Bochmann, G.; Fuchs, W.; Rittmann, S.K.M.R. Design and engineering of artificial microbial consortia for biohydrogen production. Curr. Opin. Biotechnol. 2022, 73, 74–80.
- Chong, M.L.; Rahim, R.A.; Shirai, Y.; Hassan, M.A. Biohydrogen production by Clostridium butyricum EB6 from palm oil mill effluent. Int. J. Hydrog. Energy 2009, 34, 764–771.
- Wong, Y.M.; Show, P.L.; Wu, T.Y.; Leog, H.Y.; Ibrahim, S.; Juan, J.C. Production of bio-hydrogen from dairy wastewater using pretreated landfill leachate sludge as an inoculum. J. Biosci. Bioeng. 2019, 127, 150–159.
- Greses, S.; Tomás-Pejó, E.; González-Fernández, C. Food waste valorization into bioenergy and bioproducts through a cascade combination of bioprocesses using anaerobic open mixed cultures. J. Clean. Prod. 2022, 372, 133680.
- Kumar, A.N.; Mohan, S.V. Acidogenesis of waste activated sludge—Biohydrogen production with simultaneous short chain carboxylic acids. J. Environ. Chem. Eng. 2018, 6, 2983–2991.
- Cheng, J.; Lin, R.; Xia, A.; Liu, Y.; Zhou, J.; Cen, K. Sequential Generation of Fermentative Hydrogen and Methane from Swine Manure with Physicochemical Characterization. Energy Fuel 2014, 28, 563–570.
- Wang, Y.; Wei, W.; Dai, X.; Ni, B.-J. Corncob ash boosts fermentative hydrogen production from waste activated sludge. Sci. Total Environ. 2022, 807, 151064.
- Zhang, L.; Ban, Q.; Li, J.; Zhang, S. An enhanced excess sludge fermentation process by anthraquinone-2-sulfonate as electron shuttles for the biorefinery of zero-carbon hydrogen. Environ. Res. 2022, 210, 113005.
- Litti, Y.V.; Potekhina, M.A.; Zhuravleva, E.A.; Vishnyakova, A.V.; Gruzdev, D.S.; Kovalev, A.A.; Kovalev, D.A.; Katraeva, I.V.; Parshina, S.N. Dark fermentative hydrogen production from simple sugars and various wastewaters by a newly isolated Thermoanaerobacterium thermosaccharolyticum SP-H2. Int. J. Hydrog. Energy 2022, 47, 24310–24327.
- Li, Y.-C.; Chu, C.-Y.; Wu, S.-Y.; Tsai, C.-Y.; Wang, C.-C.; Hung, C.-H.; Lin, C.-Y. Feasible pretreatment of textile wastewater for dark fermentative hydrogen production. Int. J. Hydrog. Energy 2012, 37, 15511–15517.
- Ghimire, A.; Sposito, F.; Frunzo, L.; Trably, E.; Escudié, R.; Pirozzi, F.; Lens, P.N.L.; Esposito, G. Effects of operational parameters on dark fermentative hydrogen production from biodegradable complex waste biomass. Waste Manag. 2016, 50, 55–64.
- Chang, S.; Li, J.; Liu, F. Continuous biohydrogen production from diluted molasses in an anaerobic contact reactor. Front. Environ. Sci. Eng. China 2011, 5, 140–148.
- Angeriz-Campoy, R.; Fdez-Güelfo, L.A.; Alvarez-Gallego, C.J.; Romero-García, L. Pre-composting of municipal solid wastes as enhancer of bio-hydrogen production through dark fermentation process. Fuel 2023, 333, 12657.
- Sivagurunathan, P.; Lin, C.-Y. Biohydrogen Production From Beverage Wastewater Using Selectively Enriched Mixed Culture. Waste Biomass Valorization 2020, 11, 1049–1058.
- Policastro, G.; Carraturo, F.; Compagnone, M.; Guida, M.; Fabbricino, M. Enhancing hydrogen production from winery wastewater through fermentative microbial culture selection. Bioresour. Technol. Rep. 2022, 19, 101196.
- Pachiega, R.; Rodrigues, M.F.; Rodrigues, C.V.; Sakamoto, I.K.; Varesche, M.B.A.; De Oliveira, J.E.; Maintinguer, S.I. Hydrogen bioproduction with anaerobic bacteria consortium from brewery wastewater. Int. J. Hydrog. Energy 2019, 44, 155–163.
- Ramu, S.M.; Dinesh, G.H.; Thulasinathan, B.; Rajan, A.S.T.; Ponnuchamy, K.; Pugazhendhi, A.; Alagarsamy, A. Dark fermentative biohydrogen production from rice mill wastewater. Int. J. Energy Res. 2021, 45, 17233–17243.
- Wang, J.; Yin, Y. Clostridium species for fermentative hydrogen production: An overview. Int. J. Hydrog. Energy 2021, 46, 34599–34625.
- Lertsriwong, S.; Glinwong, C. Newly-isolated hydrogen-producing bacteria and biohydrogen production by Bacillus coagulans MO11 and Clostridium beijerinckii CN on molasses and agricultural wastewater. Int. J. Hydrog. Energy 2020, 45, 26812–26821.
- Murugan, R.S.; Dinesh, G.H.; Swetha, T.R.A.; Boobalan, T.; Jothibasu, M.; Manimaran, P.S.; Selvakumar, G.; Arun, A. Acinetobacter junii AH4-A Potential Strain for Bio-hydrogen Production from Dairy Industry Anaerobic Sludge. J. Pure Appl. Microbiol. 2018, 12, 1761–1769.
- Murugan, R.S.; Dinesh, G.H.; Raja, R.K.; Obeth, E.S.J.; Bora, A.; Samsudeen, N.M.; Pugazhendhi, A.; Arun, A. Dark fermentative biohydrogen production by Acinetobacter junii-AH4 utilizing various industry wastewaters. Int. J. Hydrog. Energy 2021, 46, 11297–11304.
- Parthiba Karthikeyan, O.; Trably, E.; Mehariya, S.; Bernet, N.; Wong, J.W.C.; Carrere, H. Pretreatment of food waste for methane and hydrogen recovery: A review. Bioresour. Technol. 2018, 249, 1025–1039.
- Yang, G.; Wang, J. Kinetics and microbial community analysis for hydrogen production using raw grass inoculated with different pretreated mixed culture. Bioresour. Technol. 2018, 247, 954–962.
- Yin, T.; Wang, W.; Zhuo, S.; Cao, G.; Ren, H.; Li, J.; Xing, D.; Xie, G.; Liu, B. Thermophilic dark fermentation fast start-up of hydrogen production with substrate concentration regulation and moderate pretreatment inoculum. Fuel 2023, 334, 126748.
- Yang, G.; Wang, J. Biohydrogen production from waste activated sludge pretreated by combining sodium citrate with ultrasonic: Energy conversion and microbial community. Energy Convers. Manag. 2020, 225, 113436.
- Chen, Y.; Yin, Y.; Wang, J. Influence of butyrate on fermentative hydrogen production and microbial community analysis. Int. J. Hydrog. Energy 2021, 46, 26825–26833.
More
