Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 1 by Dario Puppi.
Flavonoids are natural compounds that are attracting great interest in the biomedical field thanks to the wide spectrum of their biological properties. Their employment as anticancer, anti-inflammatory, and antidiabetic drugs, as well as for many other pharmacological applications, is extensively investigated.
- polyphenolic compounds
- monomers
- carbonyl
1. Introduction
Flavonoids represent a class of natural products produced by plants as secondary metabolites [1] comprising more than 6000 different structures [2]. Most of them are colored substances (flavonoid from the Latin “flavus,” meaning yellow), and thus they are the source of color of leaves, flowers, and fruits of many plants along with other molecules, such as carotenoids and chlorophyll [2]. Plant flavonoids present many biological activities since they can act as signaling molecules, UV filters, and reactive oxygen species (ROS) scavengers. Moreover, they present several functional activities in drought, heat, and freezing tolerance [3], as well as in protecting plants from pathogen infection and insect feeding [4]. The biosynthesis mechanism of flavonoids in plants starts from phenylalanine, and it follows the phenylpropanoid pathway [5]. The first step involves the transformation of phenylalanine into 4-coumaroyl-CoA, which enters the flavonoid biosynthesis pathway [6]. The chalcone synthase is the first enzyme specific for the flavonoid pathway, and its role is to produce the chalcone scaffolds from which all flavonoids derive. The obtained flavonoids accumulate in vacuoles of plant cells in the form of glycosides [7]. Flavonoids induce several health benefits in humans thanks to their antioxidant, anti-inflammatory, anticancer, cardioprotective, antimicrobial, and antiviral properties [3]. For these reasons, they have been extensively studied for application in cosmetic products, as well as for biomedical and pharmacological applications. Dermatology and cosmetics are among the most common applications of flavonoids [8], for example, in the formulation of sunscreens. This is mostly due to their antiradical properties, which support their ability to absorb ultraviolet radiation in the range 250–280 nm (UVB) and 350–385 nm (UVA) [8,9][8][9]. Flavonoid encapsulation into a polymeric matrix has been extensively investigated as an effective means for their targeted and controlled release, as well as for their protection from oxidation and decomposition, which have been demonstrated to occur in aqueous-oxygenated mediums at physiological pHs [7,10,11][7][10][11]. Topical administration is a widely investigated field of application of encapsulated flavonoids aimed at exploiting the bioactivity of these compounds and more effectively penetrating the skin layers [12]. In addition, implantable devices made of biodegradable polymers and able to release flavonoids in situ have recently been investigated for guided tissue regeneration, as well as to obtain high local concentrations of the bioactive agents for prolonged periods [8,13,14][8][13][14].2. Flavonoids
The chemical structure of flavonoids is generally characterized by the presence of three rings named A, B, and C. A and B are aromatic rings, while C is a pyran ring. The A ring is fused with the C ring, and the B ring is covalently bonded to the C ring. Depending on their structural differences, in particular on the oxidation degree of the C ring, flavonoids can be divided into seven different subclasses: flavanols, flavones, isoflavones, anthocyanidins, flavanones, flavonols, and chalcones [15].
The chemical structures of the main flavonoid subclasses are depicted in Figure 1.
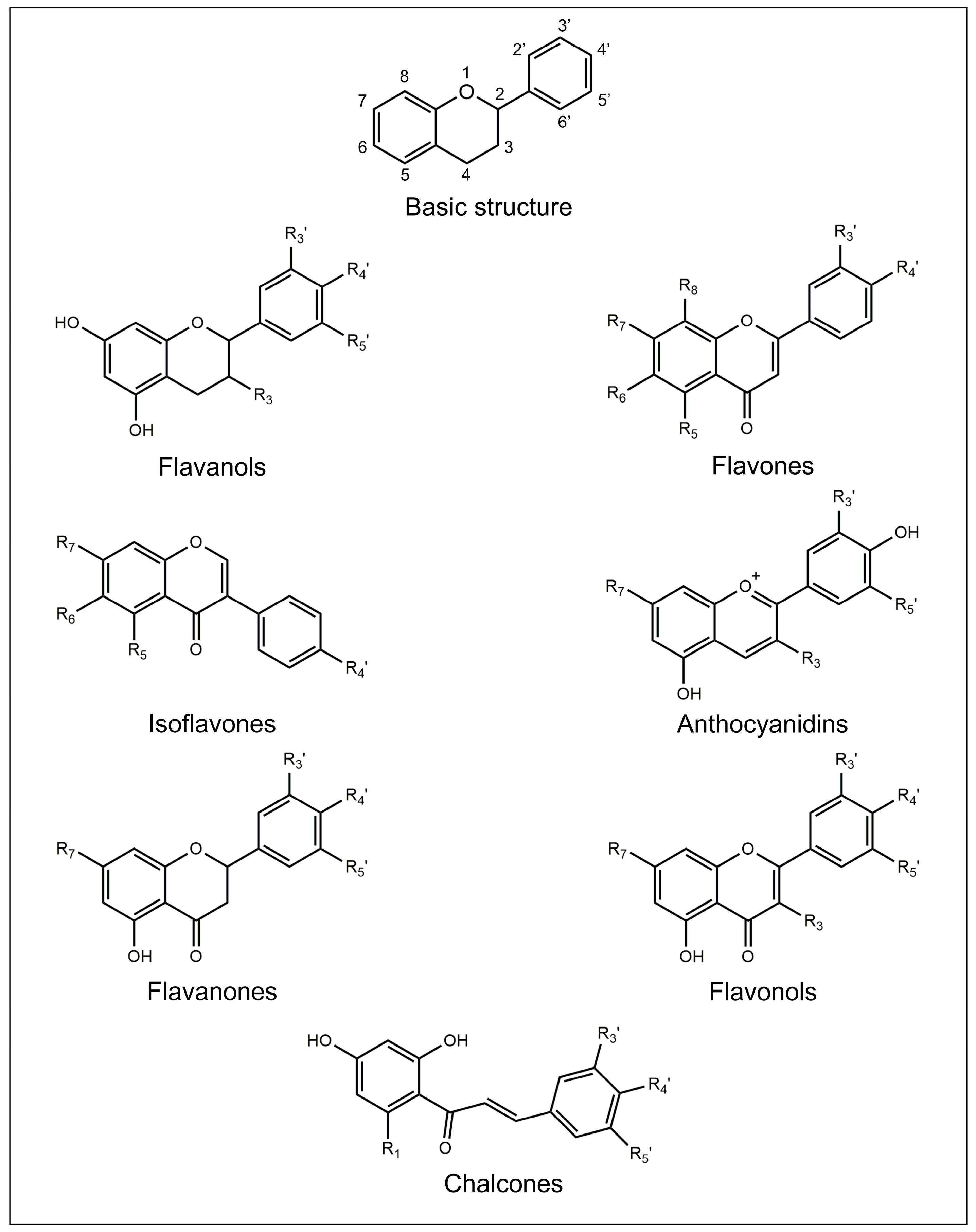
Figure 1.
Basic chemical structure of flavonoids and general molecular structures of the different flavonoid subclasses.
2.1. Flavanols
Flavanols, also known as flavan-3-ols, are characterized by a C pyran ring and a hydroxyl group bonded to the carbon in the C3 position [16]. Flavanols are usually found as aglycones, and they also exist in the form of oligomers and polymers. The monomers are called catechins and epicatechins, while the oligomers and polymers are called proanthocyanidins or condensed tannins. The monomers are differentiated by the stereochemistry of two asymmetric carbons C2 and C3, the presence of galloyl groups, as well as the level of hydroxylation of ring B [16,17][16][17]. They are naturally found in wood, bark, cereals, seeds, fruits (e.g., apples, grapes, pears, apricots, and blueberries), and beverages (e.g., chocolate, red wine, cider, tea, and beer) [16].
2.2. Flavones
Flavones are characterized by the presence of a double bond between C2 and C3 in the C ring, the absence of substitution at the C3 position, and a carbonyl group at the C4 position [18]. Together with flavonols, they are the primary pigments in white and cream-colored flowers, and they act as co-pigments with anthocyanins in blue flowers. They are generally found in plants as 7-O-glycosides, 6-C-glucosides, and 8-C-glucosides and may also have acetyl or malonyl moieties. They are commonly found in chamomile flowers, plants from the mint family, grapefruit, juice from fruits belonging to the citrus family (e.g., bergamot, mandarin orange, orange, and citron fruits), wine, olive oil, honey, vegetables (e.g., sunflower family, carrot family, parsley, chicory), cereals, and legumes (e.g., millet, sorghum) [18].
2.3. Isoflavones
The general structure of isoflavones is characterized by the B ring bonded to the C ring in the C2 position [19]. Their main role is to affect plant-microbe interactions. They show the ability to control nodulation, besides having antifungal activity and acting as precursors of phytoalexins [19]. The main sources of isoflavones are legumes from the family Fabaceae (e.g., soybean and red clover) [20].
2.4. Anthocyanidins
Anthocyanidins are aglycones species of their respective glycosides called anthocyanins. Anthocyanins chemically occur as glycosides of flavylium (2-phenylbenzopyrylium) salts differing from them by structural variations in the number of hydroxyl groups, degree of methylation of the hydroxyl groups, and the number of sugar moieties bonded to the structure [21]. They are distributed in plums, cherries, and berries of several plants, as pigments responsible for the color [21].
2.5. Flavanones
Flavanones’ general structure is characterized by the presence of a carbonyl group in the C4 position, the absence of substituents in the C3 position, and a double bond between C2 and C3 [22]. They usually occur as glycosides, usually rutinosides (6-O-α-L-rhamnosyl-D-glucosides) and neohesperidosides (2-O-α-L-rhamnosyl-D-glucosides) [23]. Flavanones are widely distributed in plants, especially in Compositae, Leguminose, and Rutaceae [22]. Citrus fruits are the major dietary source of flavanones: indeed, lemon, lime, mandarin and sweet orange are rich in rutinosides, while grapefruit and sour orange are rich in neohesperidoses [23].
2.6. Flavonols
Flavonols’ chemical structure is characterized by the presence of a carbonyl group in the C4 position, a C2-C3 double bond, and a hydroxy group bonded to the carbon in the C3 position [24]. Flavonols are mainly found in fruits and vegetables (e.g., apples and onions), as well as in beverages (e.g., red wine and teas) [25]. They are usually found in plants bound to sugars as O-glycosides [26]. They are responsible for color, taste, and the prevention of substances such as vitamins and enzymes from oxidation, as well as for protection against ultraviolet radiations and parasites [24].
2.7. Chalcones
Chalcones have a common chemical structure, 1,3-diaryl-2-propen-1-one, also known as “chalconoid” [27]. The structure exists as trans and cis isomers, with the second isomer being more thermodynamically stable than the first one. They can be found in vegetables (e.g., tomatoes), fruits (e.g., sweet oranges, apples, licorice, and Chinese ginger), and beverages (e.g., red wine, beer, coffee, and teas) [28].
2.8. Bioactivity and Bioavailability of Flavonoids
Flavonoids present many different biological activities, including antioxidant, antiaging, anti-inflammatory, immunomodulatory, cardioprotective, antimicrobial, antiviral, antibacterial, antiparasitic, antifungal, and anticancer activities [29]. According to the U.S. Food and Drug Administration (FDA), the definition of bioavailability is “the rate and extent to which the active ingredient or active moiety is absorbed from a drug product and becomes available at the site of drug action” [30]. The bioavailability of flavonoids is generally low. In addition, it usually depends on flavonoid classes, but it can also vary among individual compounds within a particular class [31]. Flavonoid bioavailability depends on many properties, such as their molecular weight, chemical structure, and degree of glycosylation, as well as the type of sugar moieties [31,32][31][32]. The solubility of flavonoids in water is generally very low; as a consequence, their absorption by oral administration is difficult, thus leading to low bioavailability and low therapeutic effect [33,34,35][33][34][35]. Furthermore, flavonoids have low stability, are easily degraded in acidic medium, and have a high metabolic rate [35,36][35][36]. Many strategies have been explored to increase flavonoid bioavailability and metabolic stability, such as the formation of microemulsions or flavonoid methylation [31], as well as their encapsulation in a drug delivery system, as one of the most promising approaches.
References
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47.
- Kasprzak, M.M.; Erxleben, A.; Ochocki, J. Properties and applications of flavonoid metal complexes. RSC Adv. 2015, 5, 45853–45877.
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377.
- Iwashina, T. Flavonoid Function and Activity to Plants and Other Organisms. Biol. Sci. Space 2003, 17, 24–44.
- Liu, W.; Feng, Y.; Yu, S.; Fan, X.; Li, X.; Li, J.; Yin, H. The Flavonoid Biosynthesis Network in Plants. Int. J. Mol. Sci. 2021, 22, 5377.
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222.
- Morales, J.; Gunther, G.; Zanocco, A.L.; Lemp, E. Singlet oxygen reactions with flavonoids. A theoretical-experimental study. PLoS ONE 2012, 7, e40548.
- Arct, J.; Pytkowska, K. Flavonoids as components of biologically active cosmeceuticals. Clin. Dermatol. 2008, 26, 347–357.
- Chen, Y.; Zhang, D.; Hu, D.; Li, X.; Luo, J.; Duan, X.; Zhang, Y.; Wang, Y. Alkaloids and flavonoids exert protective effects against UVB-induced damage in a 3D skin model using human keratinocytes. Results Chem. 2022, 4, 100298.
- Maity, S.; Acharyya, A.; Chakraborti, A. Flavonoid-based polymeric nanoparticles: A promising approach for cancer and diabetes treatment. Eur. Polym. J. 2022, 177, 111455.
- Khan, H.; Ullah, H.; Martorell, M.; Valdes, S.E.; Belwal, T.; Tejada, S.; Sureda, A.; Kamal, M.A. Flavonoids nanoparticles in cancer: Treatment, prevention and clinical prospects. Semin. Cancer Biol. 2021, 69, 200–211.
- Zverev, Y.F.; Rykunova, A.Y. Modern Nanocarriers as a Factor in Increasing the Bioavailability and Pharmacological Activity of Flavonoids. Appl. Biochem. Microbiol. 2022, 58, 1002–1020.
- Arpornmaeklong, P.; Sareethammanuwat, M.; Apinyauppatham, K.; Boonyuen, S. Characteristics and biologic effects of thermosensitive quercetin-chitosan/collagen hydrogel on human periodontal ligament stem cells. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1656–1670.
- Chen, S.; Zhu, L.; Wen, W.; Lu, L.; Zhou, C.; Luo, B. Fabrication and Evaluation of 3D Printed Poly(l-lactide) Scaffold Functionalized with Quercetin-Polydopamine for Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2019, 5, 2506–2518.
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531.
- Liu, X.; Le Bourvellec, C.; Guyot, S.; Renard, C.M.G.C. Reactivity of flavanols: Their fate in physical food processing and recent advances in their analysis by depolymerization. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4841–4880.
- Braicu, C.; Ladomery, M.R.; Chedea, V.S.; Irimie, A.; Berindan-Neagoe, I. The relationship between the structure and biological actions of green tea catechins. Food Chem. 2013, 141, 3282–3289.
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. 2017, 8, 423–435.
- Sohn, S.I.; Pandian, S.; Oh, Y.J.; Kang, H.J.; Cho, W.S.; Cho, Y.S. Metabolic Engineering of Isoflavones: An Updated Overview. Front. Plant Sci. 2021, 12, 670103.
- Krizova, L.; Dadakova, K.; Kasparovska, J.; Kasparovsky, T. Isoflavones. Molecules 2019, 24, 1076.
- Mohammed, H.A.; Khan, R.A. Anthocyanins: Traditional Uses, Structural and Functional Variations, Approaches to Increase Yields and Products’ Quality, Hepatoprotection, Liver Longevity, and Commercial Products. Int. J. Mol. Sci. 2022, 23, 2149.
- Khan, M.; Zill, E.H.; Dangles, O. A comprehensive review on flavanones, the major citrus polyphenols. J. Food Compos. Anal. 2014, 33, 85–104.
- Tomàs-Barberàn, F.A.; Clifford, M.N. Flavanones, chalcones and dihydrochalcones—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1073–1080.
- Sharma, A.; Sharma, P.; Singh Tuli, H.; Sharma, A.K. Phytochemical and Pharmacological Properties of Flavonols; John and Wiley and Sons: Hoboken, NJ, USA, 2018; pp. 1–12.
- Aherne, S.A.; O’brien, N.M. Dietary Flavonols: Chemistry, Food Content, and Metabolism. Nutrition 2002, 18, 75–81.
- Hollman, P.; Arts, I. Flavonols, flavones and flavanols—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1081–1093.
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810.
- Rudrapal, M.; Khan, J.; Dukhyil, A.A.B.; Alarousy, R.m.i.i.; Attah, E.I.; Sharma, T.; Khairnar, S.J.; Bendale, A.R. Chalcone Scaffolds, Bioprecursors of Flavonoids: Chemistry, Bioactivities, and Pharmacokinetics. Molecules 2021, 26, 7177.
- Juca, M.M.; Cysne Filho, F.M.S.; De Almeida, J.C.; Mesquita, D.D.S.; Barriga, J.R.M.; Dias, K.C.F.; Barbosa, T.M.; Vasconcelos, L.C.; Leal, L.k.a.m.; Ribeiro, J.E.; et al. Flavonoids: Biological activities and therapeutic potential. Nat. Prod. Res. 2020, 34, 692–705.
- Code of Federal Regulations. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-D/part-314/subpart-A/section-314.3 (accessed on 10 November 2022).
- Thilakarathna, S.; Rupasinghe, H. Flavonoid Bioavailability and Attempts for Bioavailability Enhancement. Nutrients 2013, 5, 3367–3387.
- Rahaman, S.; Mondal, S. Flavonoids: A vital resource in healthcare and medicine. Pharm. Pharmacol. Int. J. 2020, 8, 91–104.
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457.
- Dos Santos Lima, B.; Shanmugam, S.; De Souza Siqueira Quintans, J.; Quintans-Júnior, L.; De Souza Araújo, A. Inclusion complex with cyclodextrins enhances the bioavailability of flavonoid compounds: A systematic review. Phytochem. Rev. 2019, 18, 1337–1359.
- Ferreira, M.; Costa, D.; Sousa, A. Flavonoids-Based Delivery Systems towards Cancer Therapies. Bioengineering 2022, 9, 197.
- Mojtaba, Y.; Mahdi, S.; Sara, S.; Nasim, K.; Mortazavian, A. Encapsulation Systems for Delivery of Flavonoids: A Review. Biointerface Res. Appl. Chem. 2021, 11, 13934–13951.
More
