The p21 Activated Kinases (PAKs) are serine threonine kinases and play important roles in many biological processes, including cell growth, survival, cytoskeletal organization, migration, and morphology. Recently, PAKs have emerged in the process of liver disorders, including liver cancer, hepatic ischemia-reperfusion injury, hepatitis, and liver fibrosis, owing to their effects in multiple signaling pathways in various cell types. Activation of PAKs promotes liver cancer growth and metastasis and contributes to the resistance of liver cancer to radiotherapy and chemotherapy, leading to poor survival of patients. PAKs also play important roles in the development and progression of hepatitis and other pathological processes of the liver such as fibrosis and ischemia-reperfusion injury.
- p21 Activated Kinase
- liver cancer
- hepatitis
- liver fibrosis
1. Introduction
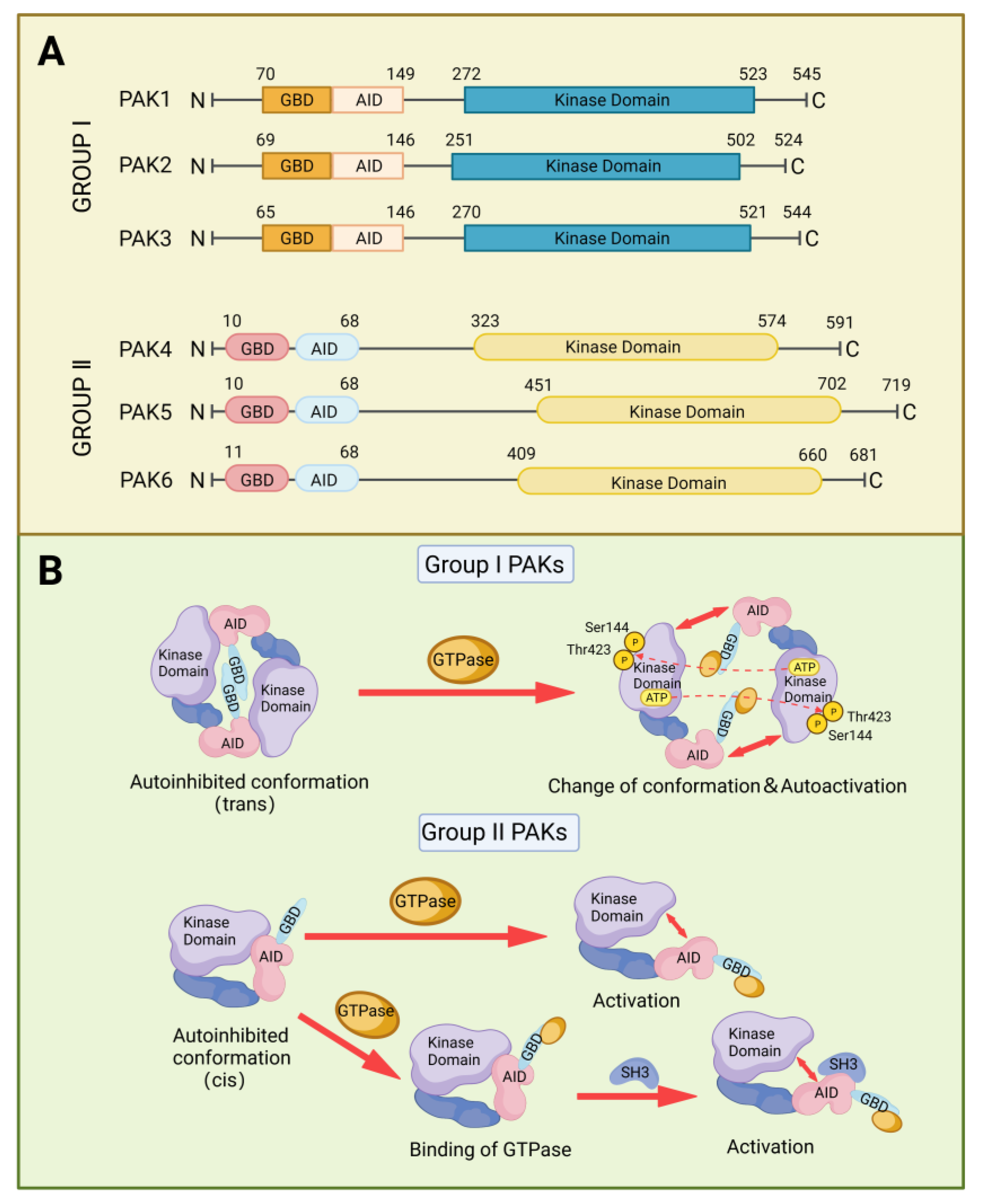
2. Structure and Activation of PAKs Family
3. PAKs in Liver Cancers
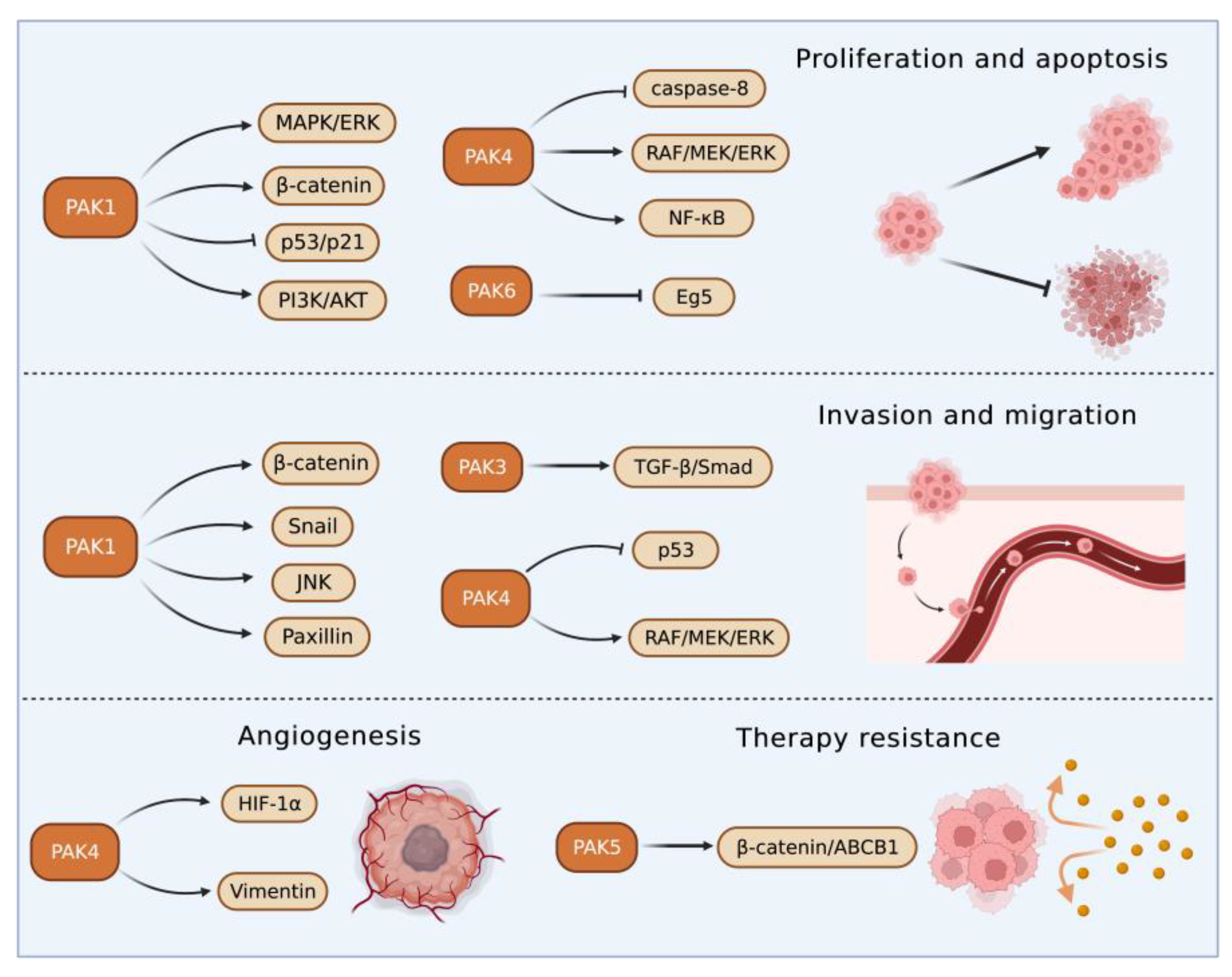
3.1. Roles of Group I PAKs in Liver Cancer
3.2. Roles of Group II PAKs in Liver Cancer
PAK4 enhanced HCC cell survival by modulating caspase-8 and NF-κB pathways [61][55]. In an HCC transgenic mouse model, miR-199-3p inhibited HCC growth by targeting the PAK4/RAF/MEK/ERK pathway [62,63][56][57]. On the contrary, an increased cyclin-dependent kinase 5 (CDK5) regulatory subunit-associated protein 3 in HCC accelerated tumor metastasis bound to and activated PAK4 [64][58]. PAK4 promoted migration and invasion of HCC cells through direct phosphorylation of p53 at S215 [65][59]. MiR-1271 suppressed HCC growth and metastasis through downregulation of RAF/MEK/ERK signaling, achieved by inhibiting Zic2/PAK4. PAK4 was overexpressed in metastatic tumor tissues compared with primary tumor and normal tissues, and PAK4 expression was correlated with worse survival of HCC patients. The results from multivariate analyses of 615 HCC patients showed that PAK4 was an independent indicator of poor prognosis [66][60]. MiR-433 was reduced in HCC tissues. MiR-433 repressed the viability of HCC cells through directly binding to the sequence at 3’-UTR of PAK4 mRNA, which in turn inhibited PAK4 expression [67][61]. MiRNAs are key upstream regulators that directly modulate the translation of multiple oncogenic proteins including PAK4. A miRNA cocktail therapy targeting PAK4, mechanistic target of rapamycin (mTOR), and RAS homolog gene family member C (RHOC), showed remarkable anti-HCC effects in patient-derived xenografts [68][62].
4. PAKs in Hepatic Ischemia/Reperfusion Injury
Hepatic IRI commonly occurs in the process of hemorrhagic shock, liver surgery, and liver transplantation [78,79][63][64]. The underlying mechanisms of hepatic IRI relate to the elevated oxidative stress and activation of immune-metabolic responses, which are harmful to normal cellular structures and functions, and result in liver damage [80][65]. PAKs participate in the modulation of immune responses and inflammation [81[66][67],82], and PAKs have been reported to play a critical role in hepatic IRI [6,83][6][68]. Neuregulin-1/PAK1 axis was found to decrease IRI of liver grafts with or without steatosis through increasing vascular endothelial growth factor-α and insulin growth factor-1 levels, respectively [83][68]. PAK4 was upregulated in hepatic IRI in mice and humans to promote hepatic hypoxia-reoxygenation-induced damage through phosphorylating nuclear factor erythroid 2-related factor 2 and suppressing its transcriptional activity [6]. Genetic knockout or pharmacological inhibition of PAK4 reduced the inflammation and necrosis of hepatocytes [6]. These results suggest that PAK4 inhibition may protect the liver from IRI-induced damage.5. PAKs in Hepatitis
Hepatitis is defined as inflammation of the liver, and its etiologies include virus infection, parasitic invasion, alcohol abuse, autoimmunity imbalance, and metabolic disorder. The main characteristic of hepatitis is represented by the infiltration of inflammatory cells, which induces apoptosis and necrosis of hepatocytes leading to liver damage and dysfunctions [84,85,86,87][69][70][71][72].
In hepatitis B, phosphorylation of PAK1 induced the translocation of RAF-1 to mitochondrial, which in turn facilitated the anti-apoptotic effect of HBV X protein in hepatocyte [88][73]. In fact, HBV X protein can activate PAK1, which protected HCC from anoikis and promoted the metastasis of HCC [56][74]. Results from the PCR showed that the PAK3 gene was the preferential integration site of HBV DNA and the integration potentially changed the expression of PAK3 [5].
Different from the promoting role of PAK1 in HBV, PAK1 had potentially inhibited HCV replication, and its antiviral effects were independent of interferon regulatory factor 3 but dependent on the mTOR-activated PI3K/AKT and ERK [91][75]. HCV protected the infected hepatocytes from cell death by suppressing apoptosis and inflammatory reaction partially through the upregulation of PAK2 [92][76].
In a mouse model infected with Schistosoma japonicum, PAK1 in Kupffer cells was elevated and facilitated the differentiation of CD4+ T cells to T helper 17 cells via the NF-κB/interferon regulatory factor 1/interleukin-6 pathway, leading to aggravated hepatic inflammation [95][77]. Increased PAK1 expression in Kupffer cells was also noticed in patients with autoimmune hepatitis, and the PAK1 expression was found to be associated with disease progression [96][78]. The increased expression of PAK6 found in alcoholic hepatitis may contribute to tumorigenesis [97][79].6. PAKs in Liver Fibrosis
Liver fibrosis is implicated in the interaction between injured hepatocytes, inflammatory cells, and hepatic myofibroblasts. In response to injury, hepatocytes will express more profibrogenic miRNAs and proteins such as TGFβ and Notch, thus initiating fibrosis [98][80]. The transformation from hepatic stellate cells to myofibroblasts that produce extracellular matrix (ECM) provides a protection to liver tissue from injury, and there is a balance between ECM production and degradation. However, the excessive accumulation of ECM produced by consistently activated myofibroblasts will impair the normal physiological structure and function of the liver, resulting in the occurrence of liver fibrosis [99][81]. PAK1 and PAK3 were upregulated in activated hepatic myofibroblasts and promoted fibrotic effects by enhancing the expression of integrin β-1, which is essential for myofibroblasts activation and ECM production [100][82].7. Therapeutic Effects of PAK Inhibitors in Liver Disorders
The main strategy for treating HBV or HCV-related liver diseases is long-term administration of antiviral drugs such as entecavir, disoproxil, and IFN-α. Different from HCV, HBV has the characteristic of escaping innate immunity, thus immune therapy is also a vital treatment for HBV [102,103][83][84]. For metabolism-related liver diseases, limiting alcohol consumption, changing eating patterns and diet composition, and reducing insulin resistance and improving lipid metabolism are effective regimens [104,105][85][86]. Liver resection and transplantation are considered as curative treatments for HCC. However, only a small proportion of patients can have these surgical operations at the diagnosis stage and the recurrence after surgery is high. Most HCC patients are diagnosed at advanced stages of the disease and can only have non-surgical treatments, including chemo-, radio-, and immune-therapies. The first-line therapy for advanced HCC includes broad-spectrum tyrosine kinase inhibitors, such as sorafenib and lenvatinib, and combinational therapies of immunotherapy and anti-angiogenesis therapy [106][87]. PAKs play key roles in liver disorders and thus are considered useful targets for the treatment of liver diseases. PAK inhibitors have been developed and can be divided into two categories depending on the binding sites within PAK molecules, ATP-competitive inhibitors, and allosteric PAK inhibitors [109][88]. ATP-competitive PAK inhibitors block PAK phosphorylation by targeting the ATP-binding pocket within the kinase domain. This type of PAK inhibitors is further classified into aminopyrazole-based inhibitors, aminopyrimidine-based inhibitors, indolocarbazole-based inhibitors, 2-amino pyrido[2,3-d] pyrimidine-7(8H)-one-based inhibitors, and other ATP-competitive inhibitors. Compared with ATP-competitive PAK inhibitors, allosteric PAK inhibitors have the potential to be more selective and display discriminative inhibitory activity among PAK family proteins [110,111][89][90]. The studies of the effects of PAK inhibitors in liver disorders are limited (Figure 43). PAK4 was upregulated and played a critical role in the process of hepatic IRI. Application of an ATP-competitive PAK4 inhibitor ND201651 reduced hepatic IRI [6]. Another novel specific PAK4 inhibitor, SPA7012, a pyrazolo [3,4-d] pyrimidine derivative, also showed an anti-inflammatory effect on the liver during hepatic IRI [112][91]. More experimental evidence is necessary for its clinical application.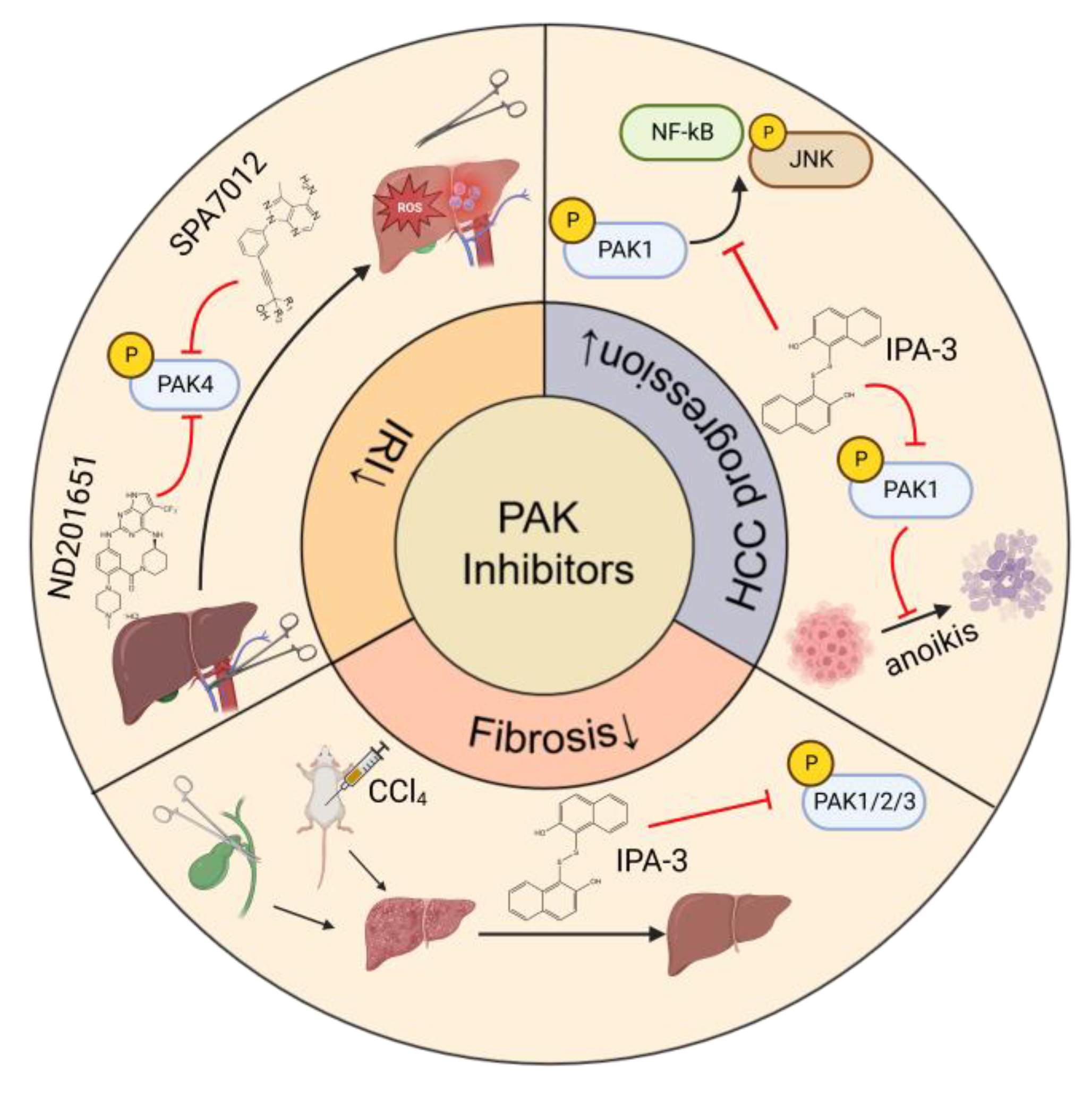
8. Conclusions
References
- Rane, C.K.; Minden, A. P21 activated kinase signaling in cancer. Semin. Cancer Biol. 2019, 54, 40–49.
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K Pathway in Human Disease. Cell 2017, 170, 605–635.
- Li, Q.; Sun, M.; Wang, M.; Feng, M.; Yang, F.; Li, L.; Zhao, J.; Chang, C.; Dong, H.; Xie, T.; et al. Dysregulation of Wnt/β-catenin signaling by protein kinases in hepatocellular carcinoma and its therapeutic application. Cancer Sci. 2021, 112, 1695–1706.
- Zhou, Q.; Gu, T.; Zhang, Y.; Li, H.; Zhuansun, X.; Xu, S.; Kuang, Y. Human Umbilical Cord Mesenchymal Stem Cells Ameliorate Hepatic Stellate Cell Activation and Liver Fibrosis by Upregulating MicroRNA-455-3p through Suppression of p21-Activated Kinase-2. BioMed Res. Int. 2021, 2021, 6685605.
- Ruan, P.; Dai, X.; Sun, J.; He, C.; Huang, C.; Zhou, R.; Chemin, I. Integration of hepatitis B virus DNA into p21-activated kinase 3 (PAK3) gene in HepG2.2.15 cells. Virus Genes 2020, 56, 168–173.
- Mao, Y.; Han, C.Y.; Hao, L.; Lee, Y.; Son, J.B.; Choi, H.; Lee, M.R.; Yang, J.D.; Hong, S.K.; Suh, K.S.; et al. p21-activated kinase 4 inhibition protects against liver ischemia/reperfusion injury: Role of nuclear factor erythroid 2-related factor 2 phosphorylation. Hepatology 2022, 76, 345–356.
- Tse, E.Y.; Ching, Y.P. The role of p21-activated kinases in hepatocellular carcinoma metastasis. J. Mol. Signal. 2014, 9, 7.
- Rane, C.K.; Minden, A. P21 activated kinases: Structure, regulation, and functions. Small GTPases 2014, 5, e28003.
- Liu, H.; Liu, K.; Dong, Z. The Role of p21-Activated Kinases in Cancer and Beyond: Where Are We Heading? Front. Cell Dev. Biol. 2021, 9, 41381.
- Baskaran, Y.; Ng, Y.; Selamat, W.; Ling, F.T.P.; Manser, E. Group I and II mammalian PAKs have different modes of activation by Cdc42. EMBO Rep. 2012, 13, 653–659.
- Ha, B.H.; Davis, M.J.; Chen, C.; Lou, H.J.; Gao, J.; Zhang, R.; Krauthammer, M.; Halaban, R.; Schlessinger, J.; Turk, B.E.; et al. Type II p21-activated kinases (PAKs) are regulated by an autoinhibitory pseudosubstrate. Proc. Natl. Acad. Sci. USA 2012, 109, 16107–16112.
- Chan, C.H.; Chiou, L.W.; Lee, T.Y.; Liu, Y.R.; Hsieh, T.H.; Yang, C.Y.; Jeng, Y.M. PAK and PI3K pathway activation confers resistance to KRAS(G12C) inhibitor sotorasib. Br. J. Cancer 2023, 128, 148–159.
- Higuchi, M.; Onishi, K.; Kikuchi, C.; Gotoh, Y. Scaffolding function of PAK in the PDK1–Akt pathway. Nature 2008, 10, 1356–1364.
- King, H.; Thillai, K.; Whale, A.; Arumugam, P.; Eldaly, H.; Kocher, H.M.; Wells, C.M. PAK4 interacts with p85 alpha: Implications for pancreatic cancer cell migration. Sci. Rep. 2017, 7, 2575.
- Park, S.Y.; An, J.M.; Seo, J.T.; Seo, S.R. Y-27632 Induces Neurite Outgrowth by Activating the NOX1-Mediated AKT and PAK1 Phosphorylation Cascades in PC12 Cells. Int. J. Mol. Sci. 2020, 21, 7679.
- Fu, X.; Feng, J.; Zeng, D.; Ding, Y.; Yu, C.; Yang, B. PAK4 confers cisplatin resistance in gastric cancer cells via PI3K/Akt- and MEK/ERK-dependent pathways. Biosci. Rep. 2014, 34, e00094.
- Huang, N.; Li, S.; Xie, Y.; Han, Q.; Xu, X.M.; Sheng, Z.H. Reprogramming an energetic AKT-PAK5 axis boosts axon energy supply and facilitates neuron survival and regeneration after injury and ischemia. Curr. Biol. 2021, 31, 3098–3114.
- Beeser, A.; Jaffer, Z.M.; Hofmann, C.; Chernoff, J. Role of Group A p21-activated Kinases in Activation of Extracellular-regulated Kinase by Growth Factors. J. Biol. Chem. 2005, 280, 36609–36615.
- Frost, J.A.; Swantek, J.L.; Stippec, S.; Yin, M.J.; Gaynor, R.; Cobb, M.H. Stimulation of NFkappa B activity by multiple signaling pathways requires PAK1. J. Biol. Chem. 2000, 275, 19693–19699.
- Zhu, M.; Xu, Y.; Zhang, W.; Gu, T.; Wang, D. Inhibition of PAK1 alleviates cerulein-induced acute pancreatitis via p38 and NF-κB pathways. Biosci. Rep. 2019, 39, BSR20182221.
- Wong, L.L.; Lam, I.P.; Wong, T.Y.; Lai, W.L.; Liu, H.F.; Yeung, L.L.; Ching, Y.P. IPA-3 inhibits the growth of liver cancer cells by suppressing PAK1 and NF-κB activation. PLoS ONE 2013, 8, e68843.
- Liu, J.; Ren, G.; Li, K.; Liu, Z.; Wang, Y.; Chen, T.; Mu, W.; Yang, X.; Li, X.; Shi, A.; et al. The Smad4-MYO18A-PP1A complex regulates β-catenin phosphorylation and pemigatinib resistance by inhibiting PAK1 in cholangiocarcinoma. Cell Death Differ. 2022, 29, 818–831.
- Yi, Y.; Li, P.; Huang, Y.; Chen, D.; Fan, S.; Wang, J.; Yang, M.; Zeng, S.; Deng, J.; Lv, X.; et al. P21-activated kinase 2-mediated β-catenin signaling promotes cancer stemness and osimertinib resistance in EGFR-mutant non-small-cell lung cancer. Oncogene 2022, 41, 4318–4329.
- Li, T.T.; Mou, J.; Pan, Y.J.; Huo, F.C.; Du, W.Q.; Liang, J.; Wang, Y.; Zhang, L.S.; Pei, D.S. MicroRNA-138-1-3p sensitizes sorafenib to hepatocellular carcinoma by targeting PAK5 mediated β-catenin/ABCB1 signaling pathway. J. Biomed. Sci. 2021, 28, 56.
- Li, Y.; Shao, Y.; Tong, Y.; Shen, T.; Zhang, J.; Li, Y.; Gu, H.; Li, F. Nucleo-cytoplasmic shuttling of PAK4 modulates β-catenin intracellular translocation and signaling. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2012, 1823, 465–475.
- Vershinin, Z.; Feldman, M.; Chen, A.; Levy, D. PAK4 Methylation by SETD6 Promotes the Activation of the Wnt/β-Catenin Pathway. J. Biol. Chem. 2016, 291, 6786–6795.
- Bastea, L.I.; Döppler, H.; Pearce, S.E.; Durand, N.; Spratley, S.J.; Storz, P. Protein kinase D-mediated phosphorylation at Ser99 regulates localization of p21-activated kinase 4. Biochem. J. 2013, 455, 251–260.
- Wang, J.; Hirose, H.; Du, G.; Chong, K.; Kiyohara, E.; Witz, I.P.; Hoon, D.S.B. P-REX1 amplification promotes progression of cutaneous melanoma via the PAK1/P38/MMP-2 pathway. Cancer Lett. 2017, 407, 6–75.
- El-Serag, H.B.; Rudolph, K.L. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology 2007, 132, 2557–2576.
- McGlynn, K.A.; London, W.T. The global epidemiology of hepatocellular carcinoma: Present and future. Clin. Liver Dis. 2011, 15, 223–243.
- Parekh, P.; Rao, K.V. Overexpression of cyclin D1 is associated with elevated levels of MAP kinases, Akt and Pak1 during diethylnitrosamine-induced progressive liver carcinogenesis. Cell Biol. Int. 2007, 31, 35–43.
- Razavi, H.; Waked, I.; Sarrazin, C.; Myers, R.P.; Idilman, R.; Calinas, F.; Vogel, W.; Mendes Correa, M.C.; Hézode, C.; Lázaro, P.; et al. The present and future disease burden of hepatitis C virus (HCV) infection with today’s treatment paradigm. J. Viral Hepat. 2014, 21 (Suppl. 1), 4–59.
- Best, M.; Gale, M.E.; Wells, C.M. PAK-dependent regulation of actin dynamics in breast cancer cells. Int. J. Biochem. Cell Biol. 2022, 146, 06207.
- Wang, M.; Zhang, C.; Zheng, Q.; Ma, Z.; Qi, M.; Di, G.; Ling, S.; Xu, H.; Qi, B.; Yao, C.; et al. RhoJ facilitates angiogenesis in glioblastoma via JNK/VEGFR2 mediated activation of PAK and ERK signaling pathways. Int. J. Biol. Sci. 2022, 18, 942–955.
- Lei, K.; Luo, M.; Tu, Z.; Lv, S.; Liu, J.; Gong, C.; Ye, M.; Wu, M.; Sheng, Y.; Long, X.; et al. Comprehensive analysis of the prognostic implications and functional exploration of PAK gene family in human cancer. Cancer Cell Int. 2022, 22, 275.
- Yu, X.; Huang, C.; Liu, J.; Shi, X.; Li, X. The significance of PAK4 in signaling and clinicopathology: A review. Open Life Sci. 2022, 17, 586–598.
- Kumar, R.; Li, D.Q. PAKs in Human Cancer Progression: From Inception to Cancer Therapeutic to Future Oncobiology. Adv. Cancer Res. 2016, 130, 37–209.
- Cao, F.; Yin, L.X. PAK1 promotes proliferation, migration and invasion of hepatocellular carcinoma by facilitating EMT via directly up-regulating Snail. Genomics 2020, 112, 694–702.
- Zhang, Z.-L.; Liu, G.-C.; Peng, L.; Zhang, C.; Jia, Y.-M.; Yang, W.-H.; Mao, L. Effect of PAK1 gene silencing on proliferation and apoptosis in hepatocellular carcinoma cell lines MHCC97-H and HepG2 and cells in xenograft tumor. Gene Ther. 2018, 25, 284–296.
- Ching, Y.P.; Leong, V.Y.; Lee, M.F.; Xu, H.T.; Jin, D.Y.; Ng, I.O. P21-activated protein kinase is overexpressed in hepatocellular carcinoma and enhances cancer metastasis involving c-Jun NH2-terminal kinase activation and paxillin phosphorylation. Cancer Res. 2007, 67, 3601–3608.
- Gujdár, A.; Sipeki, S.; Bander, E.; Buday, L.; Faragó, A. Phorbol ester-induced migration of HepG2 cells is accompanied by intensive stress fibre formation, enhanced integrin expression and transient down-regulation of p21-activated kinase 1. Cell. Signal. 2003, 15, 307–318.
- Zhang, J.G.; Zhou, H.M.; Zhang, X.; Mu, W.; Hu, J.N.; Liu, G.L.; Li, Q. Hypoxic induction of vasculogenic mimicry in hepatocellular carcinoma: Role of HIF-1 α, RhoA/ROCK and Rac1/PAK signaling. BMC Cancer 2020, 20, 32.
- Chen, L.; Liu, H.; Liu, J.; Zhu, Y.; Xu, L.; He, H.; Zhang, H.; Wang, S.; Wu, Q.; Liu, W.; et al. Klotho endows hepatoma cells with resistance to anoikis via VEGFR2/PAK1 activation in hepatocellular carcinoma. PLoS ONE 2013, 8, e58413.
- Liu, J.; Liu, H.; Zhang, W.; Wu, Q.; Liu, W.; Liu, Y.; Pan, D.; Xu, J.; Gu, J. N-acetylglucosaminyltransferase V confers hepatoma cells with resistance to anoikis through EGFR/PAK1 activation. Glycobiology 2013, 23, 1097–1109.
- Parekh, P.; Motiwale, L.; Naik, N.; Rao, K.V. Downregulation of cyclin D1 is associated with decreased levels of p38 MAP kinases, Akt/PKB and Pak1 during chemopreventive effects of resveratrol in liver cancer cells. Exp. Toxicol. Pathol. 2011, 63, 67–173.
- Iyer, S.C.; Gopal, A.; Halagowder, D. Myricetin induces apoptosis by inhibiting P21 activated kinase 1 (PAK1) signaling cascade in hepatocellular carcinoma. Mol. Cell. Biochem. 2015, 407, 223–237.
- Janardhan, H.P.; Meng, X.; Dresser, K.; Hutchinson, L.; Trivedi, C.M. KRAS or BRAF mutations cause hepatic vascular cavernomas treatable with MAP2K-MAPK1 inhibition. J. Exp. Med. 2020, 217, e20192205.
- Chen, L.; Bi, S.; Hou, J.; Zhao, Z.; Wang, C.; Xie, S. Targeting p21-activated kinase 1 inhibits growth and metastasis via Raf1/MEK1/ERK signaling in esophageal squamous cell carcinoma cells. Cell Commun. Signal. 2019, 17, 31.
- Cheng, C.; Kong, X.; Wang, H.; Gan, H.; Hao, Y.; Zou, W.; Wu, J.; Chi, Y.; Yang, J.; Hong, Y.; et al. Trihydrophobin 1 Interacts with PAK1 and Regulates ERK/MAPK Activation and Cell Migration. J. Biol. Chem. 2009, 284, 8786–8796.
- Tu, J.; Zhao, Z.; Xu, M.; Chen, M.; Weng, Q.; Ji, J. LINC00460 promotes hepatocellular carcinoma development through sponging miR-485-5p to up-regulate PAK1. Biomed. Pharmacother. 2019, 118, 109213.
- Zhang, X.; Zhang, X.; Wang, T.; Wang, L.; Tan, Z.; Wei, W.; Yan, B.; Zhao, J.; Wu, K.; Yang, A.; et al. MicroRNA-26a is a key regulon that inhibits progression and metastasis of c-Myc/EZH2 double high advanced hepatocellular carcinoma. Cancer Lett. 2018, 426, 8–108.
- Sato, M.; Matsuda, Y.; Wakai, T.; Kubota, M.; Osawa, M.; Fujimaki, S.; Sanpei, A.; Takamura, M.; Yamagiwa, S.; Aoyagi, Y. p21-activated kinase-2 is a critical mediator of transforming growth factor-β-induced hepatoma cell migration. J. Gastroenterol. Hepatol. 2013, 28, 1047–1055.
- Koth, A.P.; Oliveira, B.R.; Parfitt, G.M.; Buonocore Jde, Q.; Barros, D.M. Participation of group I p21-activated kinases in neuroplasticity. J. Physiol. 2014, 108, 70–277.
- Gao, Z.; Zhong, M.; Ye, Z.; Wu, Z.; Xiong, Y.; Ma, J.; Chen, H.; Zhu, Y.; Yang, Y.; Zhao, Y.; et al. PAK3 promotes the metastasis of hepatocellular carcinoma by regulating EMT process. J. Cancer 2022, 13, 153–161.
- Li, Q.; Zhang, X.; Wei, N.; Liu, S.; Ling, Y.; Wang, H. p21-activated kinase 4 as a switch between caspase-8 apoptosis and NF-κB survival signals in response to TNF-α in hepatocarcinoma cells. Biochem. Biophys. Res. Commun. 2018, 503, 3003–3010.
- Callegari, E.; D’Abundo, L.; Guerriero, P.; Simioni, C.; Elamin, B.K.; Russo, M.; Cani, A.; Bassi, C.; Zagatti, B.; Giacomelli, L.; et al. miR-199a-3p Modulates MTOR and PAK4 Pathways and Inhibits Tumor Growth in a Hepatocellular Carcinoma Transgenic Mouse Model. Mol. Ther. Nucleic Acids 2018, 11, 85–493.
- Hou, J.; Lin, L.; Zhou, W.; Wang, Z.; Ding, G.; Dong, Q.; Qin, L.; Wu, X.; Zheng, Y.; Yang, Y.; et al. Identification of miRNomes in human liver and hepatocellular carcinoma reveals miR-199a/b-3p as therapeutic target for hepatocellular carcinoma. Cancer Cell 2011, 19, 232–243.
- Mak, G.W.; Chan, M.M.; Leong, V.Y.; Lee, J.M.; Yau, T.O.; Ng, I.O.; Ching, Y.P. Overexpression of a novel activator of PAK4, the CDK5 kinase-associated protein CDK5RAP3, promotes hepatocellular carcinoma metastasis. Cancer Res. 2011, 71, 2949–2958.
- Xu, H.T.; Lai, W.L.; Liu, H.F.; Wong, L.L.; Ng, I.O.; Ching, Y.P. PAK4 Phosphorylates p53 at Serine 215 to Promote Liver Cancer Metastasis. Cancer Res. 2016, 76, 5732–5742.
- Lu, S.X.; Zhang, C.Z.; Luo, R.Z.; Wang, C.H.; Liu, L.L.; Fu, J.; Zhang, L.; Wang, H.; Xie, D.; Yun, J.P. Zic2 promotes tumor growth and metastasis via PAK4 in hepatocellular carcinoma. Cancer Lett. 2017, 402, 1–80.
- Xue, J.; Chen, L.Z.; Li, Z.Z.; Hu, Y.Y.; Yan, S.P.; Liu, L.Y. MicroRNA-433 inhibits cell proliferation in hepatocellular carcinoma by targeting p21 activated kinase (PAK4). Mol. Cell. Biochem. 2015, 399, 7–86.
- Shao, S.; Hu, Q.; Wu, W.; Wang, M.; Huang, J.; Zhao, X.; Tang, G.; Liang, T. Tumor-triggered personalized microRNA cocktail therapy for hepatocellular carcinoma. Biomater. Sci. 2020, 8, 6579–6591.
- Wang, M.; Zhang, J.; Gong, N. Role of the PI3K/Akt signaling pathway in liver ischemia reperfusion injury: A narrative review. Ann. Palliat. Med. 2022, 11, 806–817.
- Dery, K.J.; Kupiec-Weglinski, J.W. New insights into ischemia-reperfusion injury signaling pathways in organ transplantation. Curr. Opin. Organ Transplant. 2022, 27, 424–433.
- Gao, F.; Qiu, X.; Wang, K.; Shao, C.; Jin, W.; Zhang, Z.; Xu, X. Targeting the Hepatic Microenvironment to Improve Ischemia/Reperfusion Injury: New Insights into the Immune and Metabolic Compartments. Aging Dis. 2022, 13, 1196–1214.
- Ma, Y.; Nikfarjam, M.; He, H. The trilogy of P21 activated kinase, autophagy and immune evasion in pancreatic ductal adenocarcinoma. Cancer Lett. 2022, 24, 215868.
- Wang, K.; Zhan, Y.; Huynh, N.; Dumesny, C.; Wang, X.; Asadi, K.; Herrmann, D.; Timpson, P.; Yang, Y.; Walsh, K.; et al. Inhibition of PAK1 suppresses pancreatic cancer by stimulation of anti-tumour immunity through down-regulation of PD-L1. Cancer Lett. 2020, 472, 8–18.
- Micó-Carnero, M.; Casillas-Ramírez, A.; Sánchez-González, A.; Rojano-Alfonso, C.; Peralta, C. The Role of Neuregulin-1 in Steatotic and Non-Steatotic Liver Transplantation from Brain-Dead Donors. Biomedicines 2022, 10, 978.
- Castaneda, D.; Gonzalez, A.J.; Alomari, M.; Tandon, K.; Zervos, X.B. From hepatitis A to E: A critical review of viral hepatitis. World J. Gastroenterol. 2021, 27, 1691–1715.
- Hosseini, N.; Shor, J.; Szabo, G. Alcoholic Hepatitis: A Review. Alcohol Alcohol. 2019, 54, 408–416.
- Sucher, E.; Sucher, R.; Gradistanac, T.; Brandacher, G.; Schneeberger, S.; Berg, T. Autoimmune Hepatitis—Immunologically Triggered Liver Pathogenesis—Diagnostic and Therapeutic Strategies. J. Immunol. Res. 2019, 2019, 9437043.
- Targher, G.; Tilg, H.; Byrne, C.D. Non-alcoholic fatty liver disease: A multisystem disease requiring a multidisciplinary and holistic approach. Lancet Gastroenterol. Hepatol. 2021, 6, 578–588.
- Chen, J.; Siddiqui, A. Hepatitis B Virus X Protein Stimulates the Mitochondrial Translocation of Raf-1 via Oxidative Stress. J. Virol. 2007, 81, 6757–6760.
- Xu, J.; Liu, H.; Chen, L.; Wang, S.; Zhou, L.; Yun, X.; Sun, L.; Wen, Y.; Gu, J. Hepatitis B Virus X Protein Confers Resistance of Hepatoma Cells to Anoikis by Up-regulating and Activating p21-Activated Kinase 1. Gastroenterology 2012, 143, 199–212.e4.
- Ishida, H.; Li, K.; Yi, M.; Lemon, S.M. p21-activated kinase 1 is activated through the mammalian target of rapamycin/p70 S6 kinase pathway and regulates the replication of hepatitis C virus in human hepatoma cells. J. Biol. Chem. 2007, 282, 11836–11848.
- Nguyen, H.; Sankaran, S.; Dandekar, S. Hepatitis C virus core protein induces expression of genes regulating immune evasion and anti-apoptosis in hepatocytes. Virology 2006, 354, 58–68.
- Chang, H.; He, K.Y.; Li, C.; Ni, Y.Y.; Li, M.N.; Chen, L.; Hou, M.; Zhou, Z.; Xu, Z.P.; Ji, M.J. P21 activated kinase-1 (PAK1) in macrophages is required for promotion of Th17 cell response during helminth infection. J. Cell. Mol. Med. 2020, 24, 14325–14338.
- Lin, R.; Zhang, J.; Zhou, L.; Wang, B. Altered function of monocytes/macrophages in patients with autoimmune hepatitis. Mol. Med. Rep. 2016, 13, 3874–3880.
- Afifiyan, N.; Tillman, B.; French, B.A.; Sweeny, O.; Masouminia, M.; Samadzadeh, S.; French, S.W. The role of Tec kinase signaling pathways in the development of Mallory Denk Bodies in balloon cells in alcoholic hepatitis. Exp. Mol. Pathol. 2017, 103, 191–199.
- Kisseleva, T.; Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 2020, 18, 151–166.
- Roehlen, N.; Crouchet, E.; Baumert, T.F. Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells 2020, 9, 875.
- Martin, K.; Pritchett, J.; Llewellyn, J.; Mullan, A.F.; Athwal, V.S.; Dobie, R.; Harvey, E.; Zeef, L.; Farrow, S.; Streuli, C.; et al. PAK proteins and YAP-1 signalling downstream of integrin beta-1 in myofibroblasts promote liver fibrosis. Nat. Commun. 2016, 7, 2502.
- Odenwald, M.A.; Paul, S. Viral hepatitis: Past, present, and future. World J. Gastroenterol. 2022, 28, 1405–1429.
- Fanning, G.C.; Zoulim, F.; Hou, J.; Bertoletti, A. Therapeutic strategies for hepatitis B virus infection: Towards a cure. Nat. Rev. Drug Discov. 2019, 18, 827–844.
- Powell, E.E.; Wong, V.W.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224.
- Ganne-Carrié, N.; Nahon, P. Hepatocellular carcinoma in the setting of alcohol-related liver disease. J. Hepatol. 2019, 70, 284–293.
- Fan, Y.; Xue, H.; Zheng, H. Systemic Therapy for Hepatocellular Carcinoma: Current Updates and Outlook. J. Hepatocell. Carcinoma 2022, 9, 233–263.
- Yao, D.; Li, C.; Rajoka, M.S.R.; He, Z.; Huang, J.; Wang, J.; Zhang, J. P21-Activated Kinase 1: Emerging biological functions and potential therapeutic targets in Cancer. Theranostics 2020, 10, 9741–9766.
- Rudolph, J.; Crawford, J.J.; Hoeflich, K.P.; Chernoff, J. p21-activated kinase inhibitors. Enzymes 2013, 34, 57–180.
- Amirthalingam, M.; Palanisamy, S.; Tawata, S. p21-Activated kinase 1 (PAK1) in aging and longevity: An overview. Ageing Res. Rev. 2021, 71, 101443.
- Mao, Y.; Lee, E.; Yang, X.; Bae, E.J.; Jeon, R.; Park, B.H. Targeting p21-activated kinase 4 (PAK4) with pyrazolopyrimidine derivative SPA7012 attenuates hepatic ischaemia-reperfusion injury in mice. J. Enzyme Inhib. Med. Chem. 2022, 37, 2133–2146.
- Deacon, S.W.; Beeser, A.; Fukui, J.A.; Rennefahrt, U.E.; Myers, C.; Chernoff, J.; Peterson, J.R. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem. Biol. 2008, 15, 322–331.
