Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 1 by Maria Antonia De Francesco.
Varicella-zoster virus (VZV) is a human-specific α-herpes virus responsible for chickenpox and herpes zoster. VZV is a virus with a lipid-rich envelope acquired from cellular membranes, where viral glycoproteins are inserted. Inside the envelope, a tegument layer formed by regulatory proteins surrounds an icosahedral nucleocapsid core containing the linear double-stranded DNA genome.
- varicella-zoster virus
- pregnancy
- vaccination
21. Varicella-Zoster Virus in Pregnant Women
Varicella pneumonia is the most frequent maternal sequelae of varicella-zoster virus (VZV) primary infection, affecting 10–20% of pregnant women with chickenpox, while encephalitis and hepatitis are rare complications [3][1]. Mortality and morbidity caused by this infection are higher in pregnancy than in nonpregnant women. Specifically, if the infection occurs in the third trimester, varicella pneumonia is more severe than in other pregnancy trimesters because, in this period, the uterus is so enlarged it impacts diaphragm movements [7][2]. Before antiviral therapy, the pregnant women mortality rate ranged between 20% and 45%, while after the introduction of acyclovir and supportive care, the mortality rate decreased to 3–14% [6,8][3][4]. During the first week after the appearance of the typical varicella rash, pneumonia symptoms include fever, dry cough, exertional dyspnea, and mild hypoxemia [6,9][3][5]. Smoking, pre-existence of respiratory illnesses, immunosuppression, and the presence of more than 100 lesions represent risk factors for the development of varicella pneumonia [3,8,9][1][4][5].
Fetal sequelae depend on the gestational age at which the mother is affected by chickenpox. In particular, during the first pregnancy trimester, primary infection does not increase the risk of miscarriage [3][1]. The occurrence of VZV infection in the first and second trimesters could lead to the development of fetal varicella syndrome (FVS), also known as congenital varicella syndrome (CVS); in particular, the highest risk (2%) is documented between the 13th and 20th gestational week [6][3]. The pathogenesis of FVS consists of a herpes-zoster-like reactivation in the uterus rather than a primary infection of the fetus per se, in which fetal immature-cell-mediated immunity is not able to counteract the virus [3,6][1][3]. After 20 weeks of gestation, FVS is very rare because the developing fetus’ immune response could be able to mount a response against the virus. Infants born after FVS are affected by neurological defects, mental disorders, unilateral limb-shortening defects with muscular hypoplasia, eye diseases, cicatricial scars in dermatomal distribution, gastrointestinal and genitourinary abnormalities, and recurrent aspiration pneumonia [1[3][6],6], and 30% of infants die within the first month of life [3,7][1][2]. Maternal herpes zoster during pregnancy does not cause FVS [1,10][6][7].
The risk of fetopathy is extremely low when mothers acquire chickenpox between the 21st and the 36th gestational week, and the prognosis is favorable. Furthermore, these children are more likely to develop an uncomplicated herpes zoster in early childhood [3][1].
In the last four gestational weeks, chickenpox of the mother represents a concrete risk for the newborn to develop varicella. Indeed, perinatal infection could occur in 50% of cases, and despite passively acquired antibodies from the mother, 23% of them present varicella clinical traits. Neonatal varicella could be acquired from an ascending vaginal infection through the placenta through contact with vesicular fluid at the time of delivery, or through respiratory droplets. Nowadays, the mortality rate is 7% due to antiviral therapy, neonatal intensive supportive care, and anti-VZV immunoglobulin administration, but previously, it was 30% [3,7][1][2].
Moreover, 5 days before and 2 days after delivery, maternal VZV primary infection could result in neonatal fulminant varicella because a fetus does not have the maternal anti-VZV antibodies to overcome a high viral load [3,7][1][2]. In fact, the risk of varicella in the newborn significantly diminishes when maternal varicella occurs long enough before the term in order to allow anti-VZV transfer through the placenta. However, infants born from these mothers could present vesicular lesions or develop them within 5 days after delivery, but the prognosis is good (Figure 1) [1][6].
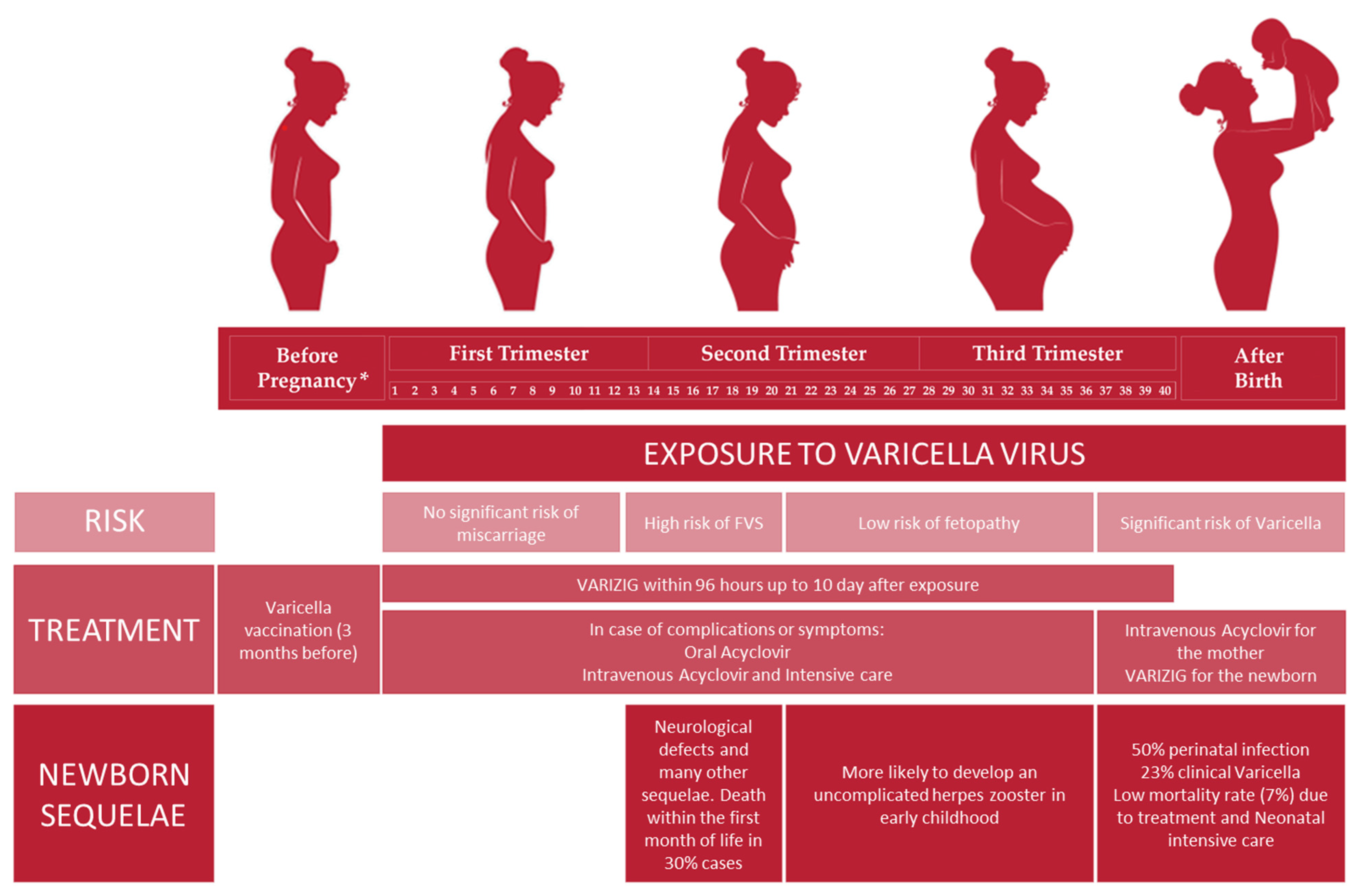
Figure 1. Management of VZV infection in pregnant seronegative women: risk, treatment and newborn sequelae related to the different pregnancy trimesters. * Before pregnancy: anti-VZV preconception screening is highly recommended in order to evaluate maternal serological status.
2. Varicella Treatment
Varicella treatment can be divided into two different approaches. The pre-exposure one mainly consists of vaccination in order to prevent the clinical manifestation of varicella and to protect those subjects who are not eligible for vaccination. The second one is the postexposure approach, which can be further divided into immunoglobulin administration and antiviral drug treatment.
2.1. Pre-Exposure Treatment
2.1.1. Varicella Vaccination
During the 1970s, the first live attenuated vaccine was developed by Takahashi et al.; this vaccine contained the Oka strain of the varicella-zoster virus passaged in guinea pig embryo fibroblasts and expanded for vaccine production in WI38 cells [1][6]. Starting from this vaccine, other companies developed several vaccines: Varivax, Varilrix and Zostavax are examples of live attenuated vaccines; Proquad and Priorix-Tetra are both live attenuated vaccines, but they consist of a combination of four different viruses; while others are under testing, such as the subunit vaccine Shingrix and the heat-inactivated vaccine V12 [4][8].
The efficacy of the currently available vaccines has been demonstrated because these vaccines induced antibody and cell-mediated responses in more than 95% of subjects enrolled in many different retrospective studies [4][8]. The cost–benefit ratio could be surely demonstrated by the decrease of 50–70% in clinical manifestations because of vaccination. According to previous studies, the immune response duration declines over years, with a still low level of antibodies detectable 10 years after vaccination [4,21][8][9]. Numerous countries adopted the two-vaccine-dose regimen, in which two doses are administered at two distant points in time to give longer protection against VZV. Adverse reactions to the varicella vaccine were registered in some small cohort studies, and most of them were only transient adverse effects, such as skin rash, pain, redness, and swelling at the inoculum site. The more severe adverse effects registered were those involving the central nervous system, with very rare cases of aseptic meningitis and cerebellar ataxia [4,22][8][10].
Since the varicella vaccine is an attenuated vaccine, reactivation may occur; similar to VZV, VZV Oka causes an infection, which is promptly contained by the immune system and, similar to the natural virus, it persists in the ganglia in a latent state [4,16][8][11]. This may lead to the reactivation of the virus, causing zoster even at a young age but boosting immunological memory [4,23][8][12].
Although studies have been conducted involving both children and adults, no efficacy analyses have been implemented concerning pregnant women. In addition, since the Varivax vaccine is a live attenuated vaccine, for 1 to 3 months postvaccination, the avoidance of pregnancy is advised [24][13].
2.1.2. Immunoglobulin
Varicella-zoster immunoglobulin is a purified immunoglobulin G preparation made from human plasma containing high levels of anti-VZV antibodies [25][14]; it is also known as VARIZIG and represents an improved version of the previous product, VZIG, which is no longer available. This preparation is recommended for high-risk groups, including pregnant women for whom varicella vaccination is not advisable. VARIZIG administration is recommended as soon as possible following VZV exposure, ideally within 96 h to, at the latest, 10 days postexposure [25,26][14][15]. The main effect of immunoglobulin preparation consists of a reduction in varicella incidence and maternal and neonatal severity symptoms [3,27][1][16]. The recommended dose of immunoglobulin is 125 units/10 kg up to a maximum of 625 units [3,28][1][17].
2.2. Postexposure Treatment
2.2.1. Vidarabine and Interferon (IFN)-Alpha
Vidarabine and IFN-α were the first antiviral agents used to treat life-threatening primary and recurrent VZV infections in immunocompromised patients [29][18]. Subsequently, these drugs were replaced by acyclovir, famciclovir, valacyclovir, and nucleoside analogs, which are currently licensed in VZV infection treatment [1][6]. Nucleoside analogs work as nucleosides during virus replication, but their structure leads to the premature termination of viral DNA polymerase activity.
2.2.2. Acyclovir
Specifically, acyclovir is a synthetic nucleoside analog of guanine [24][13], and its mechanism exploits viral and cellular kinases in order to interrupt viral replication. Acyclovir cell specificity is determined by selective phosphorylation initially operated only by the viral thymidine kinase; this particular action allows the drug to be active only in VZV-infected cells that contain this specific enzyme. After the first phosphorylation, the monophosphate form of the compound is converted into a triphosphate form, which acts as a competitive inhibitor chain terminator of viral DNA polymerase [1][6].
Acyclovir has two main limits: the first one is regarding its low bioavailability, leading to frequent oral administration to achieve therapeutic levels [3,24,30][1][13][19]. The other one consists of the fact that acyclovir is not licensed for pregnancy administration; there are potential risks of teratogenesis with acyclovir use during the first trimester [3[1][13],24], but analysis of the registries of neonates exposed to acyclovir in utero does not show any significant risk [31,32][20][21] and, moreover, data from the pregnancy registry suggest its safety [33][22]. If varicella subsequently develops, pregnant women should be immediately evaluated clinically and appropriately treated with acyclovir [34][23]. In fact, although acyclovir does not protect the fetus from FVS or the newborn from neonatal varicella, there might be some positive effects of drug migration across placenta; this is mainly due to viral replication inhibition during maternal viremia, resulting in reduced transplacental transmission and lower fetal viremia, which is one of the main responsible for FVS, together with the gestational period of infection [3,35][1][24].
Acyclovir doses depend mainly on age, symptoms, and weight. It could be given both via oral and intravenous (IV) administration: oral acyclovir dose is 800 mg/m2 five times per day [3,24][1][13] for up to 7 days, and it could be reduced to thrice a day in the case of herpes zoster reactivation [3][1]. Intravenous administration is recommended, especially in the case of clinically relevant symptoms, from rash to severe pregnancy complications, such as pneumonia [24][13]: within 24–72 h from rash onset, IV acyclovir treatment should be started with a 10–15 mg/kg of body weight dose every 8 h for 5–10 days [24][13]. The pharmacokinetics of IV-administered acyclovir results in prolonged plasma concentrations significantly above the VZV inhibitory range [1][6].
Since acyclovir has been the main drug employed to treat VZV infections and due to prolonged administration at low doses, thymidine-kinase-negative VZV mutants have been selected [1,36,37,38][6][25][26][27].
2.2.3. Valacyclovir
Valacyclovir is an acyclovir prodrug characterized by a longer half-life and better oral absorption [24,34][13][23] in comparison to acyclovir. It is a valine ester derivative of acyclovir, and immediately after absorption, it is converted into its parent compound [1,39][6][28]. Valacyclovir recommended dose is 1 g three times per day [24,30][13][19].
2.2.4. Famciclovir
Famciclovir is a penciclovir prodrug [24][13], which is a guanosine nucleoside analog [1,40,41][6][29][30]; it is uptaken by intestinal cells, and it is completed in the liver. Similar to acyclovir, it is phosphorylated by viral thymidine kinase followed by cellular thymidine kinase, resulting in a high accumulation within VZV-infected cells [1][6].
2.2.5. Other Drugs
Foscarnet and interferon-α (INF-α) are both licensed drugs to treat varicella, especially in those cases where the VZV strain belongs to acyclovir-resistant ones. These drugs show particular efficacy in high-risk patients. In addition, ganciclovir showed an in vitro activity against VZV, but it has not been tested in vivo yet due to its significant toxicity. Sorivudine, better known as BVaraU, is another nucleoside compound with particularly high inhibitory in vitro activity against VZV. It is well absorbed after oral administration, and it is recommended once or twice per day [1][6].
3. Management of Varicella Exposure in Pregnant Women
All women of childbearing age should undergo preconception screening for chickenpox. For those who have never developed VZV primary infection or do not know, a serological screening should be implemented in order to evaluate their immunological status against VZV. In case these women test negative for anti-VZV antibodies, they should be immunized before becoming pregnant [30][19]. In case of significant exposure to VZV of a pregnant woman, different pathways should be followed based on the woman’s serological status. Significant exposure to varicella means face-to-face contact or contact in the same room for 15 min or more with an affected person. Therefore, in the case of a pregnant woman, very thorough control procedures should start in order to avoid complications.
If the pregnant woman does not present a previous clinical history of chickenpox or vaccination, serology should be performed in order to evaluate her immunological status against VZV within 24–48 h from exposure. If susceptibility is confirmed, this woman should be administered with VARIZIG within 72–96 h up to 10 days after VZV exposure [3][1].
Ten days after exposure or in the case of a chickenpox rash development in a pregnant woman, the caregiver must be contacted immediately. First, symptomatic treatment with fever control and antipruritic drugs should be administered; subsequently, antiviral drug treatment should start with oral acyclovir 800 mg five times per day for 7 days. Antiviral therapy is mainly effective on the mother [3][1]; acyclovir seems to reduce fever duration and symptom severity if given to an immunocompromised subject within 24 h from rash onset [3,42][1][31]. Since there are no studies involving pregnant women, acyclovir use is not licensed but still recommended, especially in some cases; for example, when complications occur, such as respiratory symptoms, seizure, or hemorrhagic or dense rash [3[1][32],43], or if the pregnant woman is immunosuppressed, affected by chronic lung disease, or 36 weeks pregnant. If severe maternal varicella occurs, the mother should be treated with intravenous acyclovir along with supportive intensive care.
A different scenario occurs if VZV infection occurs in the last 4 weeks of pregnancy: this exposes the newborn to a significant risk of neonatal varicella [3][1].
In fact, during the viremia period with active vesicles, the delivery also carries a high risk of maternal hemorrhage and coagulopathy due to thrombocytopenia or hepatitis and a high risk of severe neonatal varicella. According to current recommendations, VARIZIG should be given to all infants born from mothers who have been affected by chickenpox from 7 days before to 7 days after delivery [3,44][1][33]; although VARIZIG does not prevent infection, it could reduce symptoms and disease severity. If the newborn develops varicella despite VARIZIG treatment, they should be administered intravenous acyclovir [45][34] (Figure 1).
References
- Nanthakumar, M.P.; Sood, A.; Ahmed, M.; Gupta, J. Varicella Zoster in pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 258, 283–287.
- Tan, M.; Koren, G. Chickenpox in pregnancy: Revisited. Reprod. Toxicol. 2006, 21, 410–420.
- Bhavsar, S.M.; Mangat, C. Congenital Varicella Syndrome ; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK568794/ (accessed on 27 December 2022).
- Baljic, R.; Hadzovic, M.; Mehanic, S.; Lukovac, E.; Koluder-Cimic, N.; Baljic, I.; Imsirovic, B. Varicella Pneumonia in a 39-year-old Female in Third Trimester Twin Pregnancy. Mater. Sociomed. 2012, 24, 16–17.
- Harger, J.H.; Ernest, J.M.; Thurnau, G.R.; Moawad, A.; Momirova, V.; Landon, M.B.; Paul, R.; Miodovnik, M.; Dombrowski, M.; Sibai, B.; et al. Risk Factors and Outcome of Varicella-Zoster Virus Pneumonia in Pregnant Women. J. Infect. Dis. 2002, 185, 422–427.
- Arvin, A.M. Varicella-zoster virus. Clin. Microbiol. Rev. 1996, 9, 361–381.
- Pupco, A.; Bozzo, P.; Koren, G. Herpes zoster during pregnancy. Can. Fam. Physician 2011, 57, 1133.
- Freer, G.; Pistello, M. Varicella-zoster virus infection: Natural history, clinical manifestations, immunity and current and future vaccination strategies. New Microbiol. 2018, 41, 95–105.
- Bennett, G.J.; Watson, C.P.N. Herpes zoster and postherpetic neuralgia: Past, present and future. Pain Res. Manag. 2009, 14, 275–282.
- Ansaldi, F.; Trucchi, C.; Alicino, C.; Paganino, C.; Orsi, A.; Icardi, G. Real-World Effectiveness and Safety of a Live-Attenuated Herpes Zoster Vaccine: A Comprehensive Review. Adv. Ther. 2016, 33, 1094–1104.
- Gershon, A.A.; Breuer, J.; Cohen, J.I.; Cohrs, R.J.; Gershon, M.D.; Gilden, D.; Grose, C.; Hambleton, S.; Kennedy, P.G.; Oxman, M.N.; et al. Varicella zoster virus infection. Nat. Rev. Dis. Primer 2015, 1, 15016.
- Brunell, P.A.; Argaw, T. Chickenpox attributable to a vaccine virus contracted from a vaccinee with zoster. Pediatrics 2000, 106, E28.
- Lamont, R.F.; Sobel, J.D.; Carrington, D.; Mazaki-Tovi, S.; Kusanovic, J.P.; Vaisbuch, E.; Romero, R. Varicella-zoster virus (chickenpox) infection in pregnancy: Chickenpox infection in pregnancy. BJOG 2011, 118, 1155–1162.
- Lachiewicz, A.M.; Srinivas, M.L. Varicella-zoster virus post-exposure management and prophylaxis: A review. Prev. Med. Rep. 2019, 16, 101016.
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of VariZIG—United States, 2013. MMWR 2013, 62, 574–576.
- Cohen, A.; Moschopoulos, P.; Stiehm, R.E.; Koren, G. Congenital varicella syndrome: The evidence for secondary prevention with varicella-zoster immune globulin. CMAJ 2011, 183, 204–208.
- Shrim, A.; Koren, G.; Yudin, M.H.; Farine, D. Management of Varicella Infection (Chickenpox) in Pregnancy. J. Obstet. Gynaecol. Can. 2018, 40, e652–e657.
- Whitley, R.J. Therapeutic approaches to varicella-zoster virus infections. J. Infect. Dis. 1992, 166 (Suppl. 1), S51–S57.
- Gardella, C.; Brown, Z.A. Managing varicella zoster infection in pregnancy. Cleve Clin. J. Med. 2007, 74, 290–296.
- Stone, K.M.; Reiff-Eldridge, R.; White, A.D.; Cordero, J.F.; Brown, Z.; Alexander, E.R.; Andrews, E.B. Pregnancy outcomes following systemic prenatal acyclovir exposure: Conclusions from the international acyclovir pregnancy registry, 1984–1999. Birth Defects Res. A Clin. Mol. Teratol. 2004, 70, 201–207.
- Pasternak, B.; Hviid, A. Use of acyclovir, valacyclovir, and famciclovir in the first trimester of pregnancy and the risk of birth defects. JAMA 2010, 304, 859–866.
- Andrews, E.B.; Yankaskas, B.C.; Cordero, J.F.; Schoeffler, K.; Hampp, S. Acyclovir in pregnancy registry: Six years’ experience. The Acyclovir in Pregnancy Registry Advisory Committee. Obstet. Gynecol. 1992, 79, 7–13.
- Daley, A.J.; Thorpe, S.; Garland, S.M. Varicella and the pregnant woman: Prevention and management. Aust. N. Z. J. Obstet. Gynaecol. 2008, 48, 26–33.
- Henderson, G.I.; Hu, Z.Q.; Johnson, R.F.; Perez, A.B.; Yang, Y.; Schenker, S. Acyclovir transport by the human placenta. J. Lab. Clin. Med. 1992, 120, 885–892.
- Jacobson, M.A.; Berger, T.G.; Fikrig, S.; Becherer, P.; Moohr, J.W.; Stanat, S.C.; Biron, K.K. Acyclovir-resistant varicella zoster virus infection after chronic oral acyclovir therapy in patients with the acquired immunodeficiency syndrome (AIDS). Ann. Intern. Med. 1990, 112, 187–191.
- Pahwa, S.; Biron, K.; Lim, W.; Swenson, P.; Kaplan, M.H.; Sadick, N.; Pahwa, R. Continuous varicella-zoster infection associated with acyclovir resistance in a child with AIDS. JAMA 1988, 260, 2879–2882.
- Safrin, S.; Berger, T.G.; Gilson, I.; Wolfe, P.R.; Wofsy, C.B.; Mills, J.; Biron, K.K. Foscarnet therapy in five patients with AIDS and acyclovir-resistant varicella-zoster virus infection. Ann. Intern. Med. 1991, 115, 119–121.
- Purifoy, D.J.M.; Beauchamp, L.M.; De Miranda, P.; Ertl, P.; Lacey, S.; Roberts, G.; Rahim, S.G.; Darby, G.; Krenitsky, T.A.; Powell, K.L. Review of research leading to new anti-herpesvirus agents in clinical development: Valaciclovir hydrochloride (256u, the L-valyl ester of acyclovir) and 882c, a specific agent for varicella zoster virus. J. Med. Virol. 1993, 41 (Suppl. 1), 139–145.
- Earnshaw, D.L.; Bacon, T.H.; Darlison, S.J.; Edmonds, K.; Perkins, R.M.; Vere Hodge, R.A. Mode of antiviral action of penciclovir in MRC-5 cells infected with herpes simplex virus type 1 (HSV-1), HSV-2, and varicella-zoster virus. Antimicrob. Agents Chemother. 1992, 36, 2747–2757.
- Vere Hodge, R.A.; Sutton, D.; Boyd, M.R.; Harnden, M.R.; Jarvest, R.L. Selection of an oral prodrug (BRL 42810; famciclovir) for the antiherpesvirus agent BRL 39123 . Antimicrob. Agents Chemother. 1989, 33, 1765–1773.
- Wallace, M.R.; Bowler, W.A.; Murray, N.B.; Brodine, S.K.; Oldfield, E.C. Treatment of adult varicella with oral acyclovir. A randomized, placebo-controlled trial. Ann. Intern. Med. 1992, 117, 358–363.
- Nathwani, D.; Maclean, A.; Conway, S.; Carrington, D. Varicella infections in pregnancy and the newborn. A review prepared for the UK Advisory Group on Chickenpox on behalf of the British Society for the Study of Infection. J. Infect. 1998, 36, 59–71.
- Chickenpox in Pregnancy (Green-top Guideline No. 13) RCOG. Available online: https://www.rcog.org.uk/guidance/browse-all-guidance/green-top-guidelines/chickenpox-in-pregnancy-green-top-guideline-no-13/ (accessed on 27 December 2022).
- Miller, E.; Cradock-Watson, J.E.; Ridehalgh, M.K. Outcome in newborn babies given anti-varicella-zoster immunoglobulin after perinatal maternal infection with varicella-zoster virus. Lancet 1989, 2, 371–373.
More
