Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Sergio Crovella.
Human monkeypox is a rare viral zoonosis that was first identified in 1970; since then, this infectious disease has been marked as endemic in central and western Africa. Questions about the necessity of developing a vaccine persist. Developing a nanomedicine-based preventative strategy might be prudent, particularly with the rapid growth of the use of nanotechnology and nanomaterials in medical research.
- monkeypox
- nanomedicine
- vaccine
1. Introduction
Monkeypox is an infectious disease caused by the monkeypox virus (MPXV), a double-stranded DNA virus belonging to the Orthopoxvirus genus (subfamily Chordopoxvirinae, family Poxviridae) [1,2][1][2]. In addition to the monkeypox virus, 11 different species of orthopoxvirus have been identified, including the camelpox virus, cowpox virus, horsepox virus, and variola virus. The vairola virus, which can cause smallpox disease, is the best-known species of this genus. It caused several epidemics throughout history before being completely eradicated in 1980 [3]. The disease was successfully eradicated by using a closely related virus, the vaccinia virus, as a smallpox vaccine [2]. Following the eradication of smallpox, monkeypox has become the most significant orthopoxvirus concern for health authorities due to occasional outbreaks in endemic countries. The clinical features of monkeypox disease are similar to those observed in smallpox-affected patients. However, the symptoms are much less severe, and in most cases, the outcome is complete recovery. Smallpox vaccines have been shown to confer immunity against the MPXV. However, the discontinuation of the national vaccine campaign made the younger population susceptible to the virus [1].
The MPXV was first isolated from Cynomolgus macaques while conducting research on polio vaccines in 1958 [4]. In 1970, a smallpox-like disease was reported in a 9-month-old boy in the Democratic Republic of the Congo, where it became endemic [5]. Since 1970, MPX outbreaks have been reported in 11 African countries, including Cameroon, Nigeria, the Central African Republic, and Gabon. The number of cases increased dramatically in endemic African countries during 2010–2019, reaching 18,000 suspected and confirmed cases. In 2003, the virus was reported for the first time in the USA, resulting in 70 cases. After that, monkeypox outbreaks outside of Africa were frequently identified in travelers from Nigeria to the United Kingdom (2018–2022), Singapore (2019), and the USA (2021) [1]. In May 2022, multiple cases of monkeypox were reported in several non-endemic countries, such as the USA, UK, France, Brazil, and others. As of 20 January 2023, the World Health Organization reported 84,916 cases and 81 deaths across 110 countries, showing a lower mortality rate than the previous outbreaks (Figure 1).
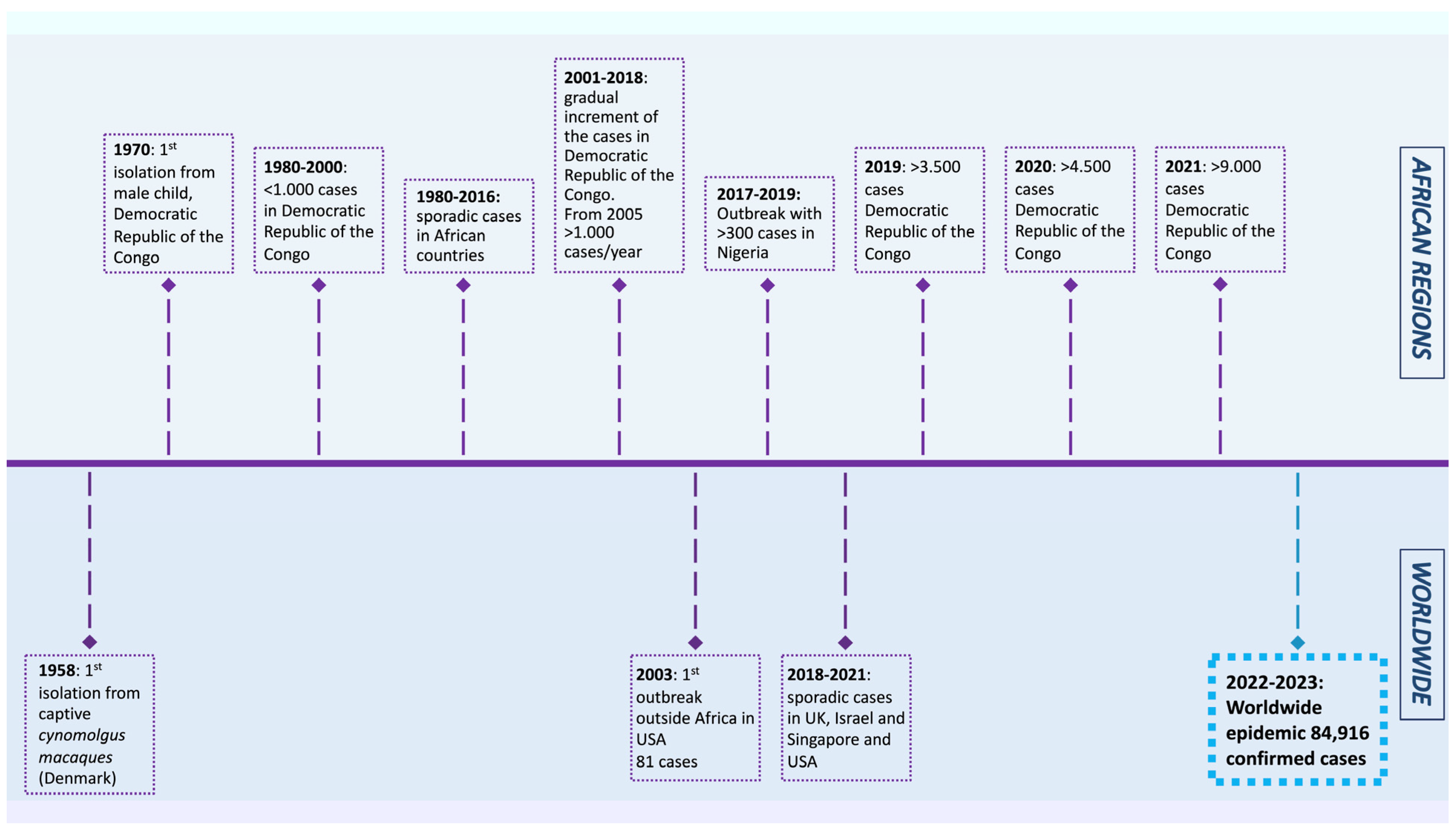
Figure 1. Timeline displaying the history of monkeypox outbreaks (1970–2023) [1,4,6,7,8,9,10,11,12].
2. Utilizing Nanomedicine Applications to Face Monkeypox
Despite the progress in developing conventional vaccines for the orthopoxvirus, improvements are still required, especially with the low immunogenicity, toxicity, instability, and multiple dose concerns associated with the conventionally available vaccines that currently employ a live attenuated vaccinia virus and not the MPXV itself or derived antigens. Furthermore, viral infections pose significant global health challenges, especially with the emergence of more resistant, rapidly evolving viruses causing new pandemics and epidemics, thus emphasizing the potential of nanomedicine applications in the field of antiviral therapies. In addition to providing delivery tools, nanomedicine can increase cellular and humoral immune responses. The involvement of nanoparticles in vaccine development can enhance the immunogenicity and stability of antigens and modulate the immune response. Therefore, over the past decades, nanoparticles such as polymers, liposomes, virus-like particles, immunostimulant complexes, inorganic nanoparticles, and emulsions have been designed and studied to stabilize vaccine antigens and act as adjuvants (Figure 42).

Figure 42.
Nanotechnology applications that could be used to face the current monkeypox epidemic.
Despite having many different nanoformulation-based vaccines, such as the virus-like particle, viral vector vaccines, and nucleic acid vaccines that are encapsulated in lipid-based nanoparticles, which are used in clinics for other viral diseases, as well as the others, including organic and inorganic nanomaterials, so far there are no nano-based vaccines in clinical use for the MPXV [56,57,58,59,60][13][14][15][16][17]. However, of the studied nanoparticles, metal-based nanoparticles have a vital role in presenting their activity without incorporating drugs. Numerous activities of metal-based nanoparticles are already well documented, such as blocking the host-virus interaction of the MPXV, competitive inhibition of the herpes simplex virus, preventing the viral binding of the hepatitis B virus, inactivating the tacaribe virus, and retarding viral attachment with the glycoprotein of the HIV-1 virus [56][13]. Furthermore, metal nanoparticles, such as iron oxide nanoparticles (FeNPs), are known to have antiviral activity, acting as viral reservoirs and chelating the virus circulating in the bloodstream, which could be used in designing detection tools for the MPXV [57][14]. Additionally, FeNPs can interfere with the different stages of the viral life cycle and modulate the immune response, as well as selectively targeting lung endothelial cells, liver cells, and spleen cells. Similar properties seen in other viruses can be tested to determine if FeNPs will have a similar effect on the MPXV. This is important as the MPXV largely affects the lung, liver, and spleen, and FeNPs are known to accumulate in these organs in addition to their anti-inflammatory activity [57,58,59,60][14][15][16][17]. Of the nanoparticle’s properties, the immunomodulatory activity is attributable to its small size, which enhances its recognition and facilitates its uptake by the antigen-presenting cells. Furthermore, the surface functionalization of nanoparticles using different moieties permits the targeted delivery of antigens to the desired cells with specific receptors, thereby stimulating selective and specific immune responses [58][15].
In the present scenario, nanoparticles (especially metals) and their unique chemical and physical properties are emerging as novel antiviral agents [61][18]. This is simply because, compared to conventional antivirals, metals can attack different virus targets, lowering the possibility of developing resistance. Moreover, metal nanoparticles have been studied for their antiviral potential and antibacterial activity against Gram-negative and Gram-positive bacteria [61][18]. Additionally, when designing nanovaccines and nanotherapeutic options for viral infections, it is important to understand the viral life cycle, as metal nanoparticles were shown to prevent or interfere with one or more stages of the viral life cycle [61][18]. This can facilitate the design of nanoagents that can interfere with one of the crucial viral life stages, such as preventing its entry into the host cell, the genome replication and assembly, the production of mature virions, and its release from the host cell. Theoretically, the antiviral activity of any metal can be tested; however, more investigations need to be made in that area.
Possible metal nanoparticles employed for this purpose include copper, zinc, titanium, magnesium, gold, alginate, and silver nanoparticles against the hepatitis B virus, respiratory syncytial virus, influenza virus, HIV-1, herpes simplex virus type 1, MPXV, and Tacaribe virus [61][18]. Interestingly, gold nanoparticles (AuNPs) were shown to have inhibitory effects on the viral infectivity and spread of the MPXV [60][17]. The uniqueness of the AuNPs depends on their resistance to tarnishing. Earlier records date the use of gold for medical purposes back to the Chinese civilization in 2500 BC. Since then, scientists have suggested utilizing Au-based nanomaterials to treat viral diseases, including smallpox, skin ulcers, measles, and syphilis [62][19]. Another nanoparticle that gained attention in the field of viral vaccine development is silver nanoparticles (AgNPs), with results showing the nanoparticles’ antiviral activity against the MPXV, hepatitis B virus, HIV, herpes simplex virus, and respiratory syncytial virus. Furthermore, other studies showed that small AgNPs (10 nm) showed a higher antiviral potency and were more effective in reducing plaque formation against the MPXV compared to larger sizes (20–80 nm) [63][20]. The AgNPs might exert such an effect by intervening during the early steps of viral binding and penetration into the host cell. Another theory is that once the virus enters the host cell, silver nanoparticles disrupt the intracellular pathways important for virus replication [64][21]. The interaction of nanoparticles with microorganisms is a developing area of research that has included evaluating the antimicrobial capacity of certain silver-containing nanoparticles against vegetative bacteria and HIV-1 [5,6][5][6]. Previous research on the nanoparticle-HIV-1 interaction demonstrated that silver-containing nanoparticles inhibited HIV-1 infectivity in vitro by binding to the disulfide bond regions of the CD4 binding domain within the gp120 glycoprotein subunit. The binding of these nanoparticles to the gp120 subunit appeared to be size-dependent, as particles larger than 10 nm were not observed attached to the viral envelope [65][22]. Based on this previous study, using silver-containing nanoparticles as an antiviral therapeutic agent may be a new area for developing nanotechnology-based antiviral therapeutics for the MPXV [65][22].
In terms of organic nanoparticles (e.g., lipid), a research group developed lipid nanoparticle (LNP)-formulated mRNA vaccines and investigated their effect on enhancing the immune response both in vitro and in vivo for use in MPXV prevention. The results showed the mRNA constructs’ translation, secretion, and biological activation, as evidenced by the increase in the target monoclonal antibody levels (c7D11, c8A, and c6C). These data demonstrated the feasibility of inducing multiple antibodies through mRNA constructs using nanotechnology [66][23]. Another study by Alec et al. developed an mRNA vaccine that encodes for four highly conserved MPXV surface proteins. These proteins are involved in the attachment, entry, transmission, and induction of MPX immunity. The vaccine was conjugated with an LNP, generating an mRNA-LNP vaccine. The results showed the superiority of the mRNA-lipid nanoparticle vaccine in neutralizing and spreading the inhibitory activity against the MPXV in tested animals compared to the lethal vaccinia virus (VACV)-treated groups. Their remarkable discovery is promising and could be a game-changer that needs further investigation [67][24]. In another study, Wang et al. employed nanoparticles in devising a diagnostic test for the MPX viral infection and the differentiation between the West and Central African MPXV isolates. The nanoparticle-based biosensor detection tool was named MPXV-MCDA-LFB, and in this test, there were two sets of multiple cross displacement amplification (MCDA) primers: D41L was designed to target the Central African MPXV, and ATI was designed to target the West African MPXV. The reaction briefly works by conducting an isothermal MCDA reaction for the DNA templates followed by the lateral flow biosensor detection of the preamplification target sequences, with the optimal reaction temperature and time being 64 °C for 30 min. The detection tool developed in this study was shown to be effective and rapid [68][25]. Moreover, the efficient inhibition of cell-pathogen interactions to prevent infections is an urgent yet unsolved problem. However, in their study, Benjamini et al. managed to develop functionalized multivalent 2D carbon nanosystems to investigate their antiviral effects. In vitro investigations that determined the ability of the orthopoxvirus strains to enter cells (Vero E6 (ECACC 85020206) and Hep2 (ECACC 86030501) showed that the 2D carbon nanosystems had excellent binding and efficient inhibition of orthopoxvirus infections. Their results suggest that these nanosystems are promising candidates to help us develop potent virus inhibitors [69][26]. Finally, a study conducted by Solenne et al. suggested inhibiting orthopoxvirus infections by blocking viral replication by using a small interfering RNA to target the D5R gene. Their study showed the potential of siRNA in treating different poxvirus infections, including smallpox and MPX. However, this will require using a carrier to ensure the safe delivery of the siRNA, which indicates the importance of investigating different nanoparticles to be used in the prevention of the MPXV [70,71][27][28].
Despite the great potential of the nanomedicine field, published work is limited to in vitro and in vivo research, while their applicability in clinical practice has yet to be achieved. However, the rapid spread of the MPXV in non-endemic countries will lay the foundation for accelerating next-generation MPX vaccines, antivirals, and diagnostics to monitor and control the MPXV [59][16].
References
- World Health Organization (WHO). Monkeypox. Available online: https://www.who.int/news-room/fact-sheets/detail/monkeypox (accessed on 8 October 2022).
- Walker, P.J.; Siddell, S.G.; Lefkowitz, E.J.; Mushegian, A.R.; Adriaenssens, E.M.; Alfenas-Zerbini, P.; Dempsey, D.M.; Dutilh, B.E.; García, M.L.; Curtis Hendrickson, R.; et al. Recent changes to virus taxonomy ratified by the International Committee on Taxonomy of Viruses (2022). Arch. Virol. 2022, 167, 2429–2440.
- World Health Organization (WHO). SmallPox. Available online: https://www.who.int/health-topics/smallpox#tab=tab_1 (accessed on 8 October 2022).
- Petersen, E.; Kantele, A.; Koopmans, M.; Asogun, D.; Yinka-Ogunleye, A.; Ihekweazu, C.; Zumla, A. Human Monkeypox: Epidemiologic and Clinical Characteristics, Diagnosis, and Prevention. Infect. Dis. Clin. N. Am. 2019, 33, 1027–1043.
- Di Giulio, D.B.; Eckburg, P.B. Human monkeypox: An emerging zoonosis. Lancet Infect. Dis. 2004, 4, 15–25.
- World Health Organization. Multi-Country Monkeypox Outbreak in Non-Endemic Countries. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON385 (accessed on 27 October 2022).
- Centers for Disease Control and Prevention. Past U.S. Cases and Outbreaks. Available online: https://www.cdc.gov/poxvirus/monkeypox/outbreak/us-outbreaks.html (accessed on 27 October 2022).
- Beer, E.M.; Rao, V.B. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl. Trop. Dis. 2019, 13, e0007791.
- European Centre for Disease Prevention and Control. Factsheet for Health Professionals on Monkeypox. Available online: https://www.ecdc.europa.eu/en/all-topics-z/monkeypox/factsheet-health-professionals (accessed on 8 October 2022).
- World Health Organization. Monkeypox—Democratic Republic of the Congo. Available online: https://web.archive.org/web/20220605103903/https://www.who.int/emergencies/disease-outbreak-news/item/monkeypox-democratic-republic-of-the-congo (accessed on 27 October 2022).
- World Health Organization. Monkeypox on the African Continent. Available online: https://cdn.who.int/media/docs/default-source/epi-win/jean-paul.pdf?sfvrsn=df67a22d_2 (accessed on 27 October 2022).
- World Health Organization. Weekly Bulletin on Outbreaks and Other Emergencies. Available online: https://apps.who.int/iris/bitstream/handle/10665/350957/OEW52-2026122021.pdf (accessed on 27 October 2022).
- Safarzadeh, M.; Sadeghi, S.; Azizi, M.; Rastegari-Pouyani, M.; Pouriran, R.; Haji Molla Hoseini, M. Chitin and chitosan as tools to combat COVID-19: A triple approach. Int. J. Biol. Macromol. 2021, 183, 235–244.
- Mohamed, N.A.; Marei, I.; Crovella, S.; Saleh, H.A. Iron-Based Nanoparticles, an accurate and powerful sniper targeting SARS-CoV-2. Adv. Cardiovasc. Res. 2020, 3, 295–304.
- Mohamed, N.A.; Davies, R.P.; Lickiss, P.D.; Ahmetaj-Shala, B.; Reed, D.M.; Gashaw, H.H.; Saleem, H.; Freeman, G.R.; George, P.M.; Wort, S.J.; et al. Chemical and biological assessment of metal organic frameworks (MOFs) in pulmonary cells and in an acute in vivo model: Relevance to pulmonary arterial hypertension therapy. Pulm. Circ. 2017, 7, 643–653.
- Mohamed, N.A.; Abou-Saleh, H.; Mohamed, H.A.; Al-Ghouti, M.A.; Crovella, S.; Zupin, L. Think like a Virus: Toward Improving Nanovaccine Development against SARS-CoV-2. Viruses 2022, 14, 1553.
- Antoine, T.E.; Mishra, Y.K.; Trigilio, J.; Tiwari, V.; Adelung, R.; Shukla, D. Prophylactic, therapeutic and neutralizing effects of zinc oxide tetrapod structures against herpes simplex virus type-2 infection. Antivir. Res. 2012, 96, 363–375.
- Kheirollahpour, M.; Mehrabi, M.; Dounighi, N.M.; Mohammadi, M.; Masoudi, A. Nanoparticles and Vaccine Development. Pharm. Nanotechnol. 2020, 8, 6–21.
- Ghosh, U.; Sayef Ahammed, K.; Mishra, S.; Bhaumik, A. The Emerging Roles of Silver Nanoparticles to Target Viral Life Cycle and Detect Viral Pathogens. Chem. Asian J. 2022, 17, e202101149.
- Kumar, A.; Mazinder Boruah, B.; Liang, X.J. Gold Nanoparticles: Promising Nanomaterials for the Diagnosis of Cancer and HIV/AIDS. J. Nanomater. 2011, 2011, 202187.
- Sharmin, S.; Rahaman, M.M.; Sarkar, C.; Atolani, O.; Islam, M.T.; Adeyemi, O.S. Nanoparticles as antimicrobial and antiviral agents: A literature-based perspective study. Heliyon 2021, 7, e06456.
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver nanoparticles as potential antiviral agents. Molecules 2011, 16, 8894.
- Rogers, J.V.; Parkinson, C.V.; Choi, Y.W.; Speshock, J.L.; Hussain, S.M. A Preliminary Assessment of Silver Nanoparticle Inhibition of Monkeypox Virus Plaque Formation. Nanoscale Res. Lett. 2008, 3, 129–133.
- Mucker, E.M.; Thiele-Suess, C.; Baumhof, P.; Hooper, J.W. Lipid nanoparticle delivery of unmodified mRNAs encoding multiple monoclonal antibodies targeting poxviruses in rabbits. Mol. Ther. Nucleic Acids 2022, 28, 847–858.
- Freyn, A.W.; Atyeo, C.; Earl, P.L.; Americo, J.L.; Chuang, G.Y.; Natarajan, H.; Frey, T.R.; Gall, J.; Moliva, J.I.; Hunegnaw, R.; et al. A monkeypox mRNA-lipid nanoparticle vaccine targeting virus binding, entry, and transmission drives protection against lethal orthopoxviral challenge. bioRxiv 2022.
- Zhou, J.; Xiao, F.; Fu, J.; Jia, N.; Huang, X.; Sun, C.; Liu, C.; Huan, H.; Wang, Y. Rapid Detection of Monkeypox Virus by Multiple Cross Displacement Amplification Combined with Nanoparticle-based Biosensor platform. J. Med. Virol. 2023, 95, e28479.
- Ziem, B.; Thien, H.; Achazi, K.; Yue, C.; Stern, D.; Silberreis, K.; Gholami, M.F.; Beckert, F.; Gröger, D.; Mülhaupt, R.; et al. Highly Efficient Multivalent 2D Nanosystems for Inhibition of Orthopoxvirus Particles. Adv. Healthc. Mater. 2016, 5, 2922–2930.
- Vigne, S.; Germi, R.; Duraffour, S.; Larrat, S.; Andrei, G.; Snoeck, R.; Garin, D.; Crance, J.M. Specific inhibition of orthopoxvirus replication by a small interfering RNA targeting the D5R gene. Antivir. Ther. 2008, 13, 357–368.
More
