Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Aysel Aslanli and Version 4 by Jessie Wu.
Toxins produced by various living organisms (bacteria, yeast, scorpions, snakes, spiders and other living organisms) are the main pathogenic factors causing severe diseases and poisoning of humans and animals. To date, recombinant forms of these toxins are widely used as antimicrobial agents, anticancer drugs, vaccines, etc. Various modifications, which in this case can be introduced into such recombinant proteins, can lead to a weakening of the toxic potency of the resulting toxins or, conversely, increase their toxicity. Thus, it is important to publicly discuss the situations and monitor the emergence of such developments.
- protein
- recombinant toxin
- antivenom
- vaccines
- killer toxins
- enzymatic antidots
1. Introduction
To date, recombinant toxins from various biological sources (bacteria, yeast, scorpions, snakes, spiders and other living organisms) are widely used as: (i) antimicrobial agents for medical purposes, as well as antimicrobial additives for the food and biotechnological industries, (ii) groundwork for the creation of drugs with anticancer activity and the treatment of neurodegenerative diseases and (iii) the basis to develop vaccines, etc. Multiple works have been performed to study the mechanisms of action of genetically modified toxins and their applications [1][2][3][4][5][6] (Figure 1).
Figure 1.
Various applications of recombinant toxins.
The protein/polypeptide nature of most of these natural toxins allows them to obtain their recombinant forms. The potential for developing these biomolecules in high enough quantities is the basis for further advancements in developing vaccines and drugs with reduced cost and their widespread use, on the one hand. On the other hand, the production of recombinant toxins avoids the need to work directly with the natural sources of these biomolecules (animals and microbial pathogens). Obtaining genetic constructs encoding the synthesis of recombinant toxins expands the possibilities of their synthesis in special modified forms. Like many recombinant proteins, recombinant toxins can be obtained in high yields using different expression systems, including extracellular secretion, and further isolated and finely purified using affine carriers [7][8].
2. Spectrum of Recombinant Toxins and Their Origins
Most of proteinaceous toxins well-studied to date are produced by various bacteria. However, toxins that are found in yeast, snake, scorpion and spider venoms and other living organisms are also actively studied by various scientific groups today. Recombinant toxins obtained from various origins and purposes of their obtaining are presented in Table 1 [9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64].
Table 1.
Recombinant toxins from various origins and the purposes of their obtaining.
| Protein | Origin | Reference |
|---|---|---|
| Production | ||
| BoNT | Bacteria Clostridium botulinum | [9] |
| Killer toxins K1, K28, K1L | Yeast Saccharomyces paradoxus | [10][11][12] |
| Killer toxin Kpkt | Yeast Tetrapisispora phaffii | [13][14] |
| ppa1, Tppa2, Tce3, Cbi1 | Scorpions of the genus Tityus and Centruroides | [15] |
| β/δ agatoxin-1 | Spider Agelena orientalis | [16] |
| Purotoxin-1 | Spiders of the genus Geolycosa sp. | [17] |
| Azemiopsin, Three-Finger Toxins | Viper Azemipos feae | [18][19] |
| MdumPLA2 | Coral snake Micrurus dumerilii | [20] |
| APHC3, HCRG21 | Sea anemone Heteractis crispa | [21][22] |
| Toxicity assays | ||
| C3bot, C3botE174Q, C2IIa | Bacteria Clostridium botulinum | [23][24][25] |
| LeTx | Bacteria Bacillus anthracis | [26] |
| HlyII | Bacteria Bacillus cereus | [27][28] |
| Cry1Ia | Bacteria Bacillus thuringiensis | [29] |
| BFT | Bacteria Bacterioides fragilis | [30] |
| EGFP-SbB, translocation domain (TD) of the diphtheria toxin |
Bacteria Corynebacterium diphtheriae | [31][32] |
| In1B | Bacteria Listeria monocytogenes | [33] |
| LcrV | Bacteria Yersinia pestis | [34] |
| AtaT2 | Bacteria Escherichia coli | [35] |
| Killer toxin Kpkt | Yeast Tetrapisispora phaffii | [36] |
| MeICT, KTx | Scorpion Mesobuthus eupeus | [37][38][39] |
| Tbo-IT2 | Spider Tibellus oblongus | [40] |
| α-conotoxins, α-cobratoxin | Marine snail and snake venom | [41] |
| Three-Finger Toxins | Viper Azemipos feae | [42] |
| α-neurotoxins | Cobra Naja melanoleuca | [43] |
| Hct-S3 | Sea anemone Heteractis crispa | [44] |
| Immunology assays | ||
| BoNT | Bacteria Clostridium botulinum | [45] |
| Beta and epsilon toxins | Bacteria Clostridium perfringens | [46][47] |
| Cholera toxin subunit B (CTB) | Bacteria Vibrio cholerae | [48] |
| Ancrod, batroxobin, RVV-V | Snakes Calloselasma rhodostoma, Bothrops atrox, Daboia russelii | [49][50] |
| Modifications | ||
| BoNT/B-MY, C2IN-C3lim | Bacteria Clostridium botulinum | [51][52] |
| DT389-YP7, s-DAB-IL-2(V6A), DT2219 | Bacteria Corynebacterium diphtheriae | [53][54][55] |
| rPA83m + plant virus spherical particles (SPs) | Bacteria Bacillus anthracis | [56][57][58] |
| SElP + Zn | Bacteria Staphylococcus aureus | [59] |
| PE38 + AgNP | Bacteria Pseudomonas aeruginosa | [60] |
| CTB-KDEL | Bacteria Vibrio cholerae | [61] |
| GFP-L2-AgTx2 | Scorpions Mesobuthus eupeus and Orthochirus scrobiculosus | [62] |
| LgRec1ALP1 | Spiders of the genus Loxosceles | [63] |
| Ms 9a-1 fragments and homologues | Sea anemone Metridium senile | [64] |
3. Diversity of Modern Purposes for Obtaining Recombinant Toxins
F2.1. Bacterial Recombinant Toxins
Bacterial cells are capable of synthesizing endo- and exotoxins. Ending ways of obtaining effectotoxins, as a rule, are cell-bound lipopolysaccharides that are released after cell destruction, while exotoxins are protein toxins that are synthesized inside cells and released into the environment. Thus, the recombinant forms of these exotoxins are discussed next (Table 1).
Botulivnum ne antibourotoxin (BoNT) and tetanus toxin (TeNT) produced by cells of the Clostridium botulinum and C. tetani, respectives and the development of vaccines againstly, are among the most dangerous and therefore the most well-studied bacterial toxins. Botulism and tetanus diseases caused by these toxins are among the most severe neurological diseases that cause flaccid paralysis and spastic paralysis, respectively. In addition, BoNT is widely used to treat a number of diseases. Consequently, recombinant tforms of these toxins is one of the main gohave been actively created and researched for many years with the aim of both developing effective antidotes and obtaining drugs based on them.
A double-blind, placebo-controls todayled study evaluated the safety, tolerability and pharmacodynamics (PD) [65][66].of the recombinant botulinum toxin Fseror maximal qutype E (rBoNT-E) compared with commercial botulinum toxin type A (ABO, Dysport ®) [9]. All doses of the recombinant toxin were wellity and effici tolerated, and rBoNT-E had a faster onset of action, a greater peak effect and a shorter duration of effect at the highest tested doses compared with ABO.
To solve ancy of immunologic medications, initial toxins sh opposite task and neutralize BoNT and other toxins, various antibodies are usually used. Special interest is afforded to single-domain camel antibodies (sdAb, VHH or nanobody) possessing unique structure and characteristics and their chimeras with usual human immunoglobulins. As a result, such immunotherapeutic agents could have up to 1000 times increased protective activity against C. botulinum and prolonged circulation in blood [45].
Different subtype highly purifs of BoNT have a varying toxicity, and BoNT/A is more potent toward the human neuroblastoma cell line as compared to BoNT/B [51]. At the same timed, be in sufficien, genetic modification of the latter to BoNT/BY resulted in improved affinity for human synaptotagmin and BoNT/B receptor, as well as increased toxicity toward this cell line.
C3 protein toxin from C. botulinum (C3bot) qucells is a mono-ADP-ribosyltrantities and stimulsferase that selectively intoxicates macrophages, osteoclasts and dendritic cells by cytosolic modification of Rho GTPases (Rho-A, Rho-B and Rho-C). Thus, C3bot and, even better, its nontoxic variant C3botE174Q have been proven as perspective transporte rs for selective immune resdelivery of small molecules, peptides and proteins to the cytosol of macrophages and other cells [23][24].
Proteolytically activated separate binding/transponrt subunit C2IIa of C2 toxin from C. botulinum has bee.n found [25] to be a Rspecombinant toxins’ productioific inhibitor of chemotaxis of polymorphonuclear neutrophils (PMN), allowing selective suppression of excessive and harmful PMN recruitment to organs as a result of trauma. The enzymatically inactive N-terminal part of the C. botulinum C2 toxin (C2IN) when fusoed to Rho-inhibiting C3 toxin from C. limosum (C3lim) significantly improves the ftoxic action of the latter [52]. In a clirnically st two issues, thoughignificant mouse model, the in vivo introduction of C2IN-C3lim into the lungs after a blunt chest injury prevented injury-induced recruitment of monocytes into the lungs. Thus, such combinatorial fusion chimeras can be of practical interest due to great variability of available toxin modules.
Until now, vaccination has bees can stiln the best way to combat diseases associated with many bacterial strains, including C. perfringens cells and α-, β- and ε-toxins of the bave cross-specificitycteria. However, commercially available vaccines are based on inactivated toxins and have many manufacturing disadvantages that can be overcome using recombinant antigens. Recombinant α-, β- and ε-toxins were synthesized in E. coli cells to create a trivalent vaccine and evaluated on rabbits, cattle, sheep and goats.
4. Prediction of Toxicity of Synthetic Recombinant Proteins
The levels of produced antibodies in all animajoritls exceeded the minimum values recommended by international protocols [46][47], thus proving the viability of the apublications of recent years emproach. Even more, nonvirulent species of the same bacteria can be modified to bear a specific toxin or its part and safely modulate strong immune response, e.g., Vibrio cholerae cells expressing thasize te β-subunit of cholera toxin (CTB) [48].Another importance of usmajor group of proteinaceous toxins is produced by the members of the genus Bacillus. Bacillus cereus cells causing foodbioinformatics methods to ideorne diseases secrete various pore-forming pathogenicity factors, including Hemolysin II (HlyII). As above mentioned, it can be specifically neutralized by antibodies [27], thus preventifng mortality nin vivo [28].
B. anthracis cewlls cause one of the most variants of toxins and clarify the mechanisms of their toxic effectsdangerous infectious diseases, Anthrax. The use of the Anthrax protective antigen (PA) is considered the most promising approach to the development of an Anthrax vaccine. However, the instability of the recombinant PA complicates the production of stable recombinant vaccines. Thus, a number of modification methods have been applied in recent years to design a stable recombinant Anthrax PA. For example, proteolytic-sensitive sites simultaneously with deamidation-prone amino acids can be genetically modified [56][57]. MAlternatively, additiolecular modenal stabilizers, e.g., spherical particles (SPs) of tobacco mosaic virus, can be added [58]. Joint applicationg facilitates the under of both methods gives even better results in terms of stability, immunogenicity and protectiveness of the final product, including in vivo tests with a fully virulent B. anthracis stranin.
B. thuringiensis cells produce δ-endotoxing os (Cry), which are toxic to a wide variety of insect pests and currently used widely in agriculture. Insertion of the interaction gene encoding Cry1Ia toxin into a bacterial strain inhibiting fungal growth results in combined fungistatic and insecticidal activity as well as ability to induce plant resistance [29].
A lot of tbacterial toxins within their receptors and/or tstructures contain metal ions performing various purposes. First of all, metal ions can be located in the active sites of metalloproteases such as BFT toxin from B. fragilis leading to damage and necrgetsosis of the intestinal epithelium [30]. In addition, such mespecially when these comtals can contribute to toxin structural stabilization and even promote recognition of the target receptor like in the case of staphylococcal enterotoxin-like protein P (SElP) from Staphylococcus aureus binding to major histocompatibility complex class II (MHCII) [59]. It should be nds are boundoted that some virulence factors secreted by bacteria may be toxic to the membrane, and biochemicmicroorganisms themselves. To prevent collateral damage and to additionally protect active components, they can be secreted in nanovesicles, which are able to be modeled in silico [30].
The diphtheria toxin (DT) from Corynebacterium diphtheriae kills approaches to the study of these processes are complexmammalian cells by inactivating the elongation factor EF-2. The translocation domain in DT plays a critical role in allowing the catalytic domain to pass to the cytosol from endosomal compartments and can be used as a functional vector for active transport of protein drugs [6731].
Some mammalian species are resistant to Due to advances in synthetic biology, the cost and time requiT. The DT receptor, proHB-EGF, in resistant and sensitive species differs by amino acid sequence and therefore by secondary structure; however, there is no consensual opinion on how the difference in the structure of primary receptors changes the process of internalization of DT by resistant cells compared to sensitive ones. According to some publications [32], there can be even very little d for the developmeifference of binding constants of DT subunit B (which includes receptor-binding and translocation domains) to resistant and synthesis of individual recombinantensitive cells, while there was huge difference of intracellular concentrations of toxin within model cells. It means that multiple mechanisms of resistance to DT may exist in mammalian cells.
Several aproducts are steadily decreaproved drugs, e.g., denileukin diftitox, which is fusion of DT with interleukin-2, are commercially available and actively used to date. However, research to improve their efficiency, producibility and safety as well as to obtain new therapeutics with DT are constantly continued [54][55].
Listeria monocytogenes cells apply ing. Many research laboratories regulaternalins InlA and InlB to attach and penetrate into mammalian cells. Curiously, hepatocyte growth factor receptors (HGFR) together with other multiple variants are also affected by InlB [33]. This is important since HGF/HGFR ply create genetically modified proteiay crucial role in liver restoration after its acute toxic damage. Thus, truncated bacterial InlB was implemented as a functional analogue of HGF to obtain novel drugs with hepatoprotective activity.
Bacterial toxins cas a part of their rn interact not only with receptors themselves but with complexes of receptor and signal molecules. One of such examples is LcrV from Yersinia pestis [34]. It is a strong virulesncearch activities. However, manipulations of factor having multiple functionalities, one of which is specific activation of human receptor-bound interferon-γ (hIFN-γ), which resulted in immune cell death via apoptosis. It became possible only after hIFN-γ binding to receptor and presentation of its 138GRRA141 site, which specificamlly ino aciteracts with 32LEEL35 and/or 203DEEI206 sitequences in pros of LcrV. Thus, inactivation of these sites by specific antibodies completely prevents any harmful effects of LcrV.
Protein bios can lead to the unintendedynthesis can be targeted by bacterial toxins, as well. For example, bacteria can utilize multiple enzymes from Gcn5-related N-acetyltransferase (GNAT) superfamily to acetylate and thus inactivate specific aminoacyl tRNAs, including transporters of Met, Ile, Gly, etc. [35].
2.2. Yeast Recombinant Toxins
Killer yeasts are able to produce protion of protein toxinseins named “killer toxins” that are often glycosylated and bind to specific receptors on the surface of the sensitive microorganism, which is then destroyed by a target-specific mechanism of action (Table 1). They arefore, the abi widespread among yeasts and attract a lot of attention of researchers. To date, more than 100 types of killer yeasts have been described [65]. The most well-characterity to zed killer toxins in terms of their genetic determine the toxiants, biochemical characteristics, molecular targets on sensitive cells and mechanisms of their destruction are toxins K1, K2 and K28 from Saccharomyces cerevisiae; zymocin from Kluyveromyces lactis; PMKT and PMKT2 from Pichia membranifaciens; PaKT from Wickerhamomyces anomalus; HM-1 from Cyberlindnera mrakii and Kpkty from Tetrapisispora phaffii. Due to their properties and spectrum of action, protein before its synthesis reduces the riwhich is aimed at pathogenic microorganisms, recombinant killer toxins are being actively investigated in order to develop therapeutic agents based on them. However, the lack of research on their effects on humans and animals limits their use in the food and feed industry. Another drawback is that additional information about the mechanisms underlying the formation of killer toxins in yeast is required. Without solving these issues, it is not possible to successfully implement killer toxins in practice [65][66].
A study of S. paradoxus revealed a new K1-like of the ptoxin (K1L) being active against sensitive competing yeast cells [10]. It is encoded by double-stential dangranded RNA (dsRNA) and satellite dsRNA, which may also be of virus origin. Its homologues have been identified in other six yeast species not belonging to Saccharomyces and are likely to be acquir of synthetic proded by horizontal gene transfer via dsRNA and/or DNA with subsequent diversification of their structure and toxicity profile.
Genetic fusion of toxins with fluoresction of pent proteins allowed researchers to study the binding of the toxin to the cell envelope of affected yeast [11]. However, intracellular transloteincation of labeled recombinant toxins. For this purpose, various methods based on machine learning a K28 was not observed then, in spite of the presence of toxicity. It means there are gaps in our understanding of the true mechanism of killer toxin action and transport even among best-investigated ones. Further research is required to visualize intracellular transfer of toxins using high-resolution imaging techniques of individual molecules.
Killer toxin K1 is se bcreted by S. cerevisiae strains in a heteroding developmeric form. After binding to the primary receptor (β-1,6 glucans) in the cell wall, K1 is transported to predict the toxicity of proteins in sithe plasma membrane and is initially supposed to interact with its secondary receptor Kre1p, which ultimately leads to an ionophoric disruption of the membrane function. However, expression of recombinant K1α in resistant yeasts lacking Kre1p resulted in profound toxic effect [12], thus excludico based on a number of initial datang role of the receptor. At the same time, co-expression of toxin precursor(s) in sensitive cells eliminated any negative effects. Thus, resistance to killer toxins is a part of adaptive (Figure 2acquired) immune system.
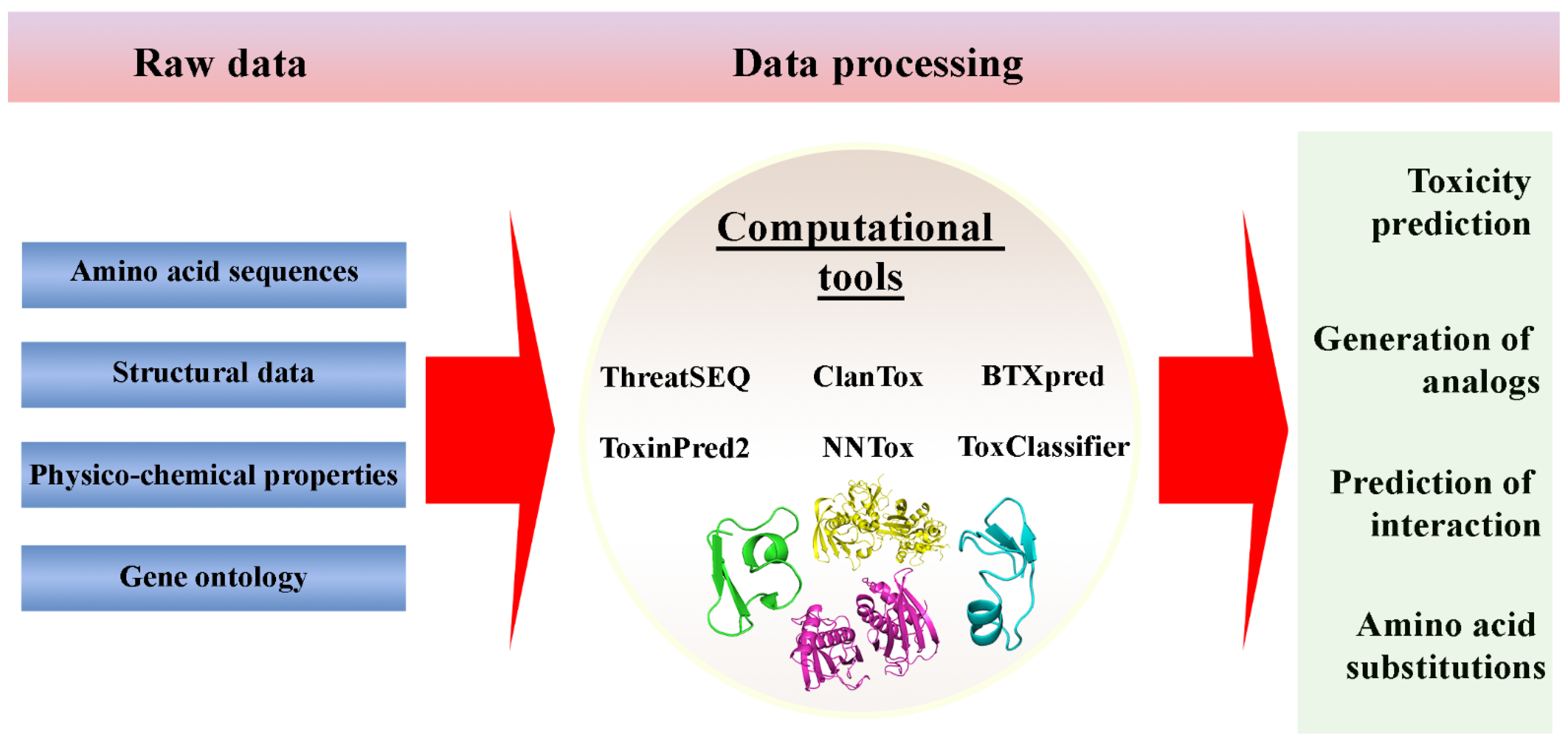

Figure 2. Machine-learning methods based tools for protein toxicity prediction.
5. Potential Enzymatic Antidotes for Recombinant Toxins
Du
Some killer to txins, e.g., Kpkt from T. phaffii (formerly Kluyveromyces phaffii), have wide variantimicrobial activity not only on yeast but also on bacteria [13]. Interesty of toxiingly, activity of Kpkt was not detected toward all tested mycelial fungi. Meanwhile, Kpkt has a β-1,3-glucanase activity [14][36] and thus known to date and differences in the meccan be combined, for example, with chitinases to synergistically improve their antifungal effects. At concentrations effective again yeasts, recombinant Kpkt has no effect on immortalized human epidermal keratinocyte cell line HaCaT [36]. That makes it promisinisg for further investigations.
2.3. Recombinant Toxins of Various Animals
Venoms of snakes, scorpions and spiders are used by animals as their action, there is an urgent need to create antidotes that both have a specific effect and are active against a wide range of toxinsown defensive and offensive means by immobilizing victim and blocking the functional activity of their cardiovascular, respiratory and/or nervous systems. Proteinaceous toxins are the main components of these systems and modulate important ion channels and receptors after introduction into the body. Today, powerful databases of poisons and protein toxins with improved properties have been assembled already for more selective action, resistance to the effects of proteases, less immunogenicity and improved characteristics, in terms of pharmacokinetic properties. These characteristics can be improved by genetic modification of amino acid sequences, addition of disulfide and ion bridges, etc. After all, animal venom toxins are of great interest for applications in medicine as a basis for drug development [5] (Table 1).
The mβ/δ again dtoxin-1 of the spider Agelena orientalis was obtained in rectionsombinant form in the entomopathogenic fungus Lecanicillium muscarium with a special secretof anry signal peptide [16]. Further toxidote development n was fused with eGFP to simplify the screening procedure. Unfortunately, toxic activity of the fusion protein was not investigated in the work.
Another fusiodayn protein of GFP with agitoxin-2 from scorpion Leiurus quinquestriatus hebraeus ware s more useful [62]. That allowed researchers to vither the creationsualize the binding of toxins to their receptor as well as to determine dissociation constants of various inhtoxins competing for the same Kv1.3 channel.
Purotoxin-1 (PT1) from the venom of the Central Asian spider Geolycosa sp. selectively inhibits the purinergic receptors capable of blocking the P2X3 and is a potent analgetic. It can be produced in pilot scale as self-cleavable fusion protein with mini-intein DnaB [17]. However, its purifites of bindcation is multistage and labor-intensive with modest yield at the end.
Interestingly, ofTbo-IT2 toxins t was identified in the spider Tibellus oblongus by cDNA analysis of targhe transcriptome of its venom glands [40]. Its amino acid sequence has a 41% idents or the production of proteins (usually antibodies) city match with the closest protein toxin, while its spatial structure folds into a well-known inhibitory cysteine knot (ICK). The first main difference is the formation of five disulfide bonds instead of the typical three that should result in extreme stability of the toxin. The second and the most puzzling difference is that Tbo-IT2 did not have inhibitory activity on the tested panel of available ion channels and neuroreceptors, while it is still toxic to the housefly, Musca domestica. Further reseaprch mable of y elucidate the target(s) of the Tbo-IT2.
Another acttempt to apply mini-inting as bioscein DnaB was a little bit more successful [21], although the target toxin, APHC3 from the anemone H. crispa, which has analgesic activity, was producengers vd in inclusion bodies and multistage purification was still required.
Fusion with His-tag and Smt3-leader peptide wa binds shown to be a much more efficient method [22]. The resulting inhibitor of the TRPV1 ion channel (HCRG21 peptide from the sea anemone H. magnifica) was easierectly to to purify and after cleavage was obtained at comparable yield to APHC3.
As stated previously withe bacterial toxins thems, antibodies are used almost exclusively in antivenoms [20]. Combining several recombinant toxins in simple mixture [15][49] or even in fus,ion protein [63] ofthereby limiting their interactions with targeten leads to improved efficiency of antivenoms, including comparing to commercial ones. Furthermore, it was found that rationally selected toxin-specific single-stranded DNA aptamers can exhibit broad cross-reactivity in vitro and ex vivo against isoforms of toxins found in various snake venoms [6850].
Computer Hmodeling provides powever, the searcrful tools to thoroughly solve even complicated issues. For example, interaction of proteinaceous toxins KTx from scorpion M. eupeus with potand development ofssium channels (KV) was simulated and explained, followed by modulation of their activity using genetic modification [38][39]. In othewr work [43], authors have intidotes basevestigated binding of TFTs with novel receptors. Secondary structures of multiple actinoporins Hct from the sea anemone Heteractis crispa were generated [67], followed by an othealysis and successful structure–activity hypothesis testing.
Venoms contain a large number of biologically principactive compounds with diverse activities. Shorter peptides, e.g., azemiopsin acting on neuroreceptors [18] and bradykinin-potentiating peptides (i.e., affecting blsood pressure) [19], ncould be prepamelyred by a solid phase synthesis using molecules cap a general Fmoc-method, while larger polypeptides are the most rational to produce using common expression systems [42][43].
A modulabltory effe of detoxifyingct of some proteinaceous toxins on neuroreceptors is worth mentioning. Well-known three-finger toxins (TFTs) and their analogues [42][43] as well as azemiopsin by their enzyind mostly nicotinic acetylcholine receptors (nAChRs), but γ-aminobutyric acid receptors (GABARs) can also be affected [43].
Pore-formaing tic tranoxins, e.g., Hct from the sea anemone H. crispa [44], have a wide nonspeciformation into less toxic or nic action and are almost equally cytotoxic to normal and malignant cells. However, fusing them with targeting partners, such as site-specific ligands, toxins or antibodies, could result in new drug platform development.
Recombintoxic molecules, may become a promant toxins can be easily genetically modified and truncated to help researchers investigate their toxic action in a more detailed way. For example, peptide Ms 9a-1 from sea anemone Metridium senile causes significant analgesing alternative to existing solutionsc and anti-inflammatory effects by desensitization of TRPA1-expressing sensory neurons, and it was thought to be a positive modulator of TRPA1 channel. However, truncation of its unordered domains on the N- or C-terminus resulted in complete loss of analgesic and anti-inflammatory activities in vivo [64]. Thus, ano date, severther target receptor(s) is likely present in neurons.
2.4. Recombinant Prions
Prions (Pr) alre enzymes are infectious agents that cause devastating and incurable disorders known that can act as antitoxins against various bacterial toxic substances, as well as enzymes that exhibit hyas transmissible spongiform encephalopathies (TSE). With the advent of innovative technologies, such as protein misfolding cyclic amplification (PMCA) and real-time quaking-induced conversion (RT-QuIC), in vitro amplification of prions has become possible. There is evidence suggesting that prion complexes can acquire high-order assemblies in vivo, which may look structurally ordered. However, the biophysical nature of these structures and their role in amyloid biology are still unclear. Despite the fact that the amyloid collected in vitro has some biochemical similarities with the ex vivo amyloid of the same protein, it often does not reproduce the biological activity of the latter. For example, preparations of prion protein (PrP), which are resistant to proteinase K and obtained exclusively from one recombinant PrP (rPrP), may not have any detectable infectious activity both in cell cultures and in animal bioassays. However, the proteinase K-resistant PrP obtained from rPrP is infectious if it is placed in the homogenate of a diseased brain ex vivo using the PMCA assay [68].
To study the rProlytic activity against PrPP, mechanisms of the development of toxicity and pathogenicity of prion diseases as well as their role in the development of pathologies of the nervous system is an important task of the world scientific community (Table 2, [69][70][71][72][73][74][75][76][77]).
Table 2. Enzymes as antidotes for toxic and prion proteins.
Recombinant prion proteins and purposes of their biosynthesis and use.
| Recombinant Protein | EPurpose of Biosynthesis anzymd Use | MReferechanism of Actionnce | Reference |
|---|---|---|---|
| GrPrP from a bacuanylyltransferase TglT frolovirus-insect cell expression system Mycobacterium tuberculosis(Bac-rPrP) | STo deterine protein kmine whether Bac-rPrPSc is sponase TakAtaneously produced in intermittent ultrasonic reactions |
Specifically, phosphorylates the cognate toxin at residue S78, thereby neutralizing toxicity[69] | [69] |
| HMousepT toxin from PrP (MoPrP, residues 89–230) in complex with Shewanella oneidensisa nanobody (Nb484) | MTo understand the role of the hydrophobic region in forming inimafectious prion at the molecular l nucleotidyltransferase (MNT)evel |
MNT acts as an adenylyltransferase and mediates the transfer of three AMPs to a tyrosine residue next to the RNase domain of HepT[70] |
[70] |
| BTracterial GhoT toxinnsgenic mice expressing elk PrP (TgElk) | ETo test vaccine candoribonuclidates against chronic wasting disease | GhoS is a sequence-specific endoribonuclease that cleaves mRNA encoding GhoT, preventing its translation[71] | [71] |
| HharPrP toxin fromfrom bank vole (BV Escherichia colirPrP) | AnTo develop a method oxygen-dependento dry and preserve the prion protein for long term st antitoxin TomBorage |
Inactivation of the Hha by oxidation with molecular oxygen mediated by the TomB[72] | [72] |
| Mycobacterium tuberculosis toxin DarTMurine rPrP | DarG—DTo study how RNA ADP-ribosyl glycohydrolas can influence the aggregation of the murine rPrP | DarG could reverse the DNA ADP-ribosylation by DarT[73] | [73] |
| PrP from the brains of clinically sick mice that had been intracerebrally inoculated with the Rocky Mountain Laboratory (RML) prion strain | CTommercially availabl demonstrate that prions are not directly neurotoxic and that toxicity present in infected brain tissue can be distinguished from infe subtilisin enzyme, Prionzymectious prions |
Proteolytic inactivation/degradation[74] | [74] |
| BV rPrP (amino acids 23–231) | STo ubtilisin 309 and Subtilisinderstand how different cofactors modulate prion strain generation and selection 309-v | Proteolytic inactivation/degradation[75] | [75] |
| PrP | Nattokinase (NK, also known as subtilisin NAT) produced by Bacillus subtilis natto | NK is capable of decreasing amyloid structure of recombinant human PrP fibrils |
[76] |
| PrP | Keratinase KerA from B. licheniformis PWD-1 |
Proteolytic inactivation/degradation | [77] |
Recent studies have shown that the infectivity of prions and their neurotoxicity may not be related to each other. Therefore, it is important to distinguish directly infective prions and those with a toxic effect, since the current hypothesis suggests that it is not the prions themselves that are toxic but another type of protein responsible for the toxicity of the disease. This species may be a by-product of prion formation, in a non-pathway amyloid PrP structure or even a non-protein whose formation is catalyzed by a prion [76]. Thus, using highly purified infectious prions, it was demonstrated that prions are not directly neurotoxic and that the toxicity presented in infected brain tissue may be different from infectious prions [74].
rPrP was obtained using the insect baculovirus cell expression system (Bac-rPrP) [69] to determine whether pathogenic Bac-pathogenic PrP (PrPSc) is produced spontaneously in intermittent ultrasound reactions. No spontaneous formation of Bac-rPrPSc was observed at 37 °C, but when the reaction temperature increased to 45 °C, Bac-rPrPSc was formed in all samples studied. Some variants of Bac-rPrPSc were transmitted to mice, but when the reaction was repeated for 40 cycles, transmissibility was lost. It is noteworthy that various variants of Bac-rPrPSc, including nontransmissive ones, were characterized by resistance to proteinase K and were dependent on the presence of cofactors during amplification. However, their characteristics also disappeared after 40 reaction cycles, and the variety converged on one variant. These results show that different variants of Bac-rPrPSc are generated with different transmissivity to mice and structural properties; variants of Bac-rPrPSc compete with each other and gradually converge to a variant with a slightly higher amplification rate.
To understand the role of the hydrophobic region in the formation of an infectious prion at the molecular level, X-rays of crystal structures of mouse PrP (MoPrP, residues 89–230) in complex with a nanobody (Nb484) were obtained [70]. Using a rPrP reproduction system, it has been shown that binding of Nb484 to the hydrophobic region of MoPrP effectively inhibits the reproduction of proteinase-resistant PrPSc and the infectivity of prions. In addition, when added to cultured mouse brain slices in high concentrations, Nb484 did not exhibit neurotoxicity, which is sharply different from other neurotoxic antibodies against PrP. Thus, Nb484 may be a potential therapeutic agent against prion disease.
Five groups of transgenic mice expressing elk PrP (TgElk) were vaccinated with either one CpG adjuvant or one of four rPrP immunogens: deer dimer (Ddi); deer monomer (Dmo); mouse dimer (Mdi) and mouse monomer (Mmo) [71]. Then mice were intraperitoneally infected with prions of chronic wasting disease (CWD). All vaccinated mice developed anti-PrP antibody titers detected by ELISA. It is important to note that all four vaccinated groups survived longer than the control group, while in the group immunized with Mmo, the average survival time increased by 60% compared to the control group (183 vs. 114 days after inoculation).
Thus, the use of recombinant forms of prions allows researchers to study their immunogenicity and to develop novel vaccines.
In order to establish how various cofactors modulate the formation and selection of prion strains, PMCA was used to generate a variety of infectious rPrP strains by multiplication in the presence of brain homogenate [75]. It is known that brain homogenate contains certain cofactors whose identity is only partially known and which facilitate the transformation of normal PrP (PrPC) into PrPSc. A mixture of various infectious prion strains was obtained and introduced into the brain homogenate, where various polyanionic cofactors were present. These cofactors could control the evolution of mixed prion populations toward the development of specific strains (types of conformations). As a result, it has been shown that various infectious rPrP can be obtained in vitro. Their specific conformation (strain) depends on the cofactors available during reproduction.
These observations are very important for understanding the pathogenesis of prion diseases and their ability to reproduce in various tissues and hosts.
The RT-QuIC method can be used to detect pathogenic PrP in various biological tissues of humans and animals. However, this method requires a continuous supply of freshly purified PrP and thus is not available in a diagnostic laboratory. To solve the issue, a method for obtaining a rPrP has been developed [72]. Lyophilized rPrP from bank vole (BV rPrP) can be stored for a long time before use, as well as be transported at certain temperatures to appropriate diagnostic laboratories, which can facilitate implementation of the RT-QuIC method as a diagnostic tool [72].
Nucleic acids have been shown in recent studies to act as potential cofactors of protein aggregation and prionogenesis. For example, RNAs, regardless of their sequence, source and size, modulate rPrP aggregation in a bimodal manner, affecting both the degree and the rate of rPrP aggregation depending on the concentration [73].
References
- Kondakova, O.A.; Nikitin, N.A.; Evtushenko, E.A.; Ryabchevskaya, E.M.; Atabekov, J.G.; Karpova, O.V. Vaccines against anthrax based on recombinant protective antigen: Problems and solutions. Expert Rev. Vaccines 2019, 18, 813–828. Micoli, F.; Bagnoli, F.; Rappuoli, R.; Serruto, D. The role of vaccines in combatting antimicrobial resistance. Nat. Rev. Microbiol. 2021, 19, 287–302.
- Fleming, B.D.; Ho, M. Generation of single-domain antibody-based recombinant immunotoxins. In Single-Domain Antibodies; Humana: New York, NY, USA, 2022; Volume 2446, pp. 489–512. Shilova, O.; Shramova, E.; Proshkina, G.; Deyev, S. Natural and designed toxins for precise therapy: Modern approaches in experimental oncology. Int. J. Mol. Sci. 2021, 22, 4975.
- Doxey, A.C.; Mansfield, M.J.; Montecucco, C. Discovery of novel bacterial toxins by genomics and computational biology. Toxicon 2018, 147, 2–12. Marzhoseyni, Z.; Shojaie, L.; Tabatabaei, S.A.; Movahedpour, A.; Safari, M.; Esmaeili, D.; Mahjoubin-Tehran, M.; Jalili, A.; Morshedi, K.; Khan, H.; et al. Streptococcal bacterial components in cancer therapy. Cancer Gene Ther. 2022, 29, 141–155.
- Aruwa, C.E.; Mukaila, Y.O.; Ajao, A.A.-N.; Sabiu, S. An appraisal of antidotes’ effectiveness: Evidence of the use of phyto-antidotes and biotechnological advancements. Molecules 2020, 25, 1516. Khatuntseva, E.A.; Nifantiev, N.E. Cross reacting material (CRM197) as a carrier protein for carbohydrate conjugate vaccines targeted at bacterial and fungal pathogens. Int. J. Biol. Macromol. 2022, 218, 775–798.
- Yu, X.; Gao, X.; Zhu, K.; Yin, H.; Mao, X.; Wojdyla, J.A.; Qin, B.; Huang, H.; Wang, M.; Sun, Y.-C.; et al. Characterization of a toxin-antitoxin system in Mycobacterium tuberculosis suggests neutralization by phosphorylation as the antitoxicity mechanism. Commun. Biol. 2020, 3, 216. Wulff, H.; Christophersen, P.; Colussi, P.; Chandy, K.G.; Yarov-Yarovoy, V. Antibodies and venom peptides: New modalities for ion channels. Nat. Rev. Drug. Discov. 2019, 18, 339–357.
- Yao, J.; Zhen, X.; Tang, K.; Liu, T.; Xu, X.; Chen, Z.; Guo, Y.; Liu, X.; Wood, T.K.; Ouyang, S.; et al. Novel polyadenylylation-dependent neutralization mechanism of the HEPN/MNT toxin/antitoxin system. Nucleic Acids Res. 2020, 48, 11054–11067. Oliveira, L.V.; Wang, R.; Specht, C.A.; Levitz, S.M. Vaccines for human fungal diseases: Close but still a long way to go. Vaccines 2021, 6, 33.
- Wang, X.; Lord, D.M.; Cheng, H.Y.; Osbourne, D.O.; Hong, S.H.; Sanchez-Torres, V.; Quiroga, C.; Zheng, K.; Herrmann, T.; Peti, W.; et al. A new type V toxin-antitoxin system where mRNA for toxin GhoT is cleaved by antitoxin GhoS. Nat. Chem. Biol. 2012, 8, 855–861. Geron, M. Production and purification of recombinant toxins. In Snake and Spider Toxins; Humana: New York, NY, USA, 2020; Volume 2068, pp. 73–84.
- Marimon, O.; Teixeira, J.M.; Cordeiro, T.N.; Soo, V.W.; Wood, T.L.; Mayzel, M.; Amata, I.; Garcia, J.; Morera, A.; Gay, M.; et al. An oxygen-sensitive toxin-antitoxin system. Nat. Commun. 2016, 7, 13634. Rodrigues, R.R.; Ferreira, M.R.A.; Kremer, F.S.; Donassolo, R.A.; Júnior, C.M.; Alves, M.L.F.; Conceição, F.R. Recombinant vaccine design against Clostridium spp. toxins using immunoinformatics tools. In Vaccine Design; Humana: New York, NY, USA, 2022; Volume 2012, pp. 457–470.
- Jankevicius, G.; Ariza, A.; Ahel, M.; Ahel, I. The toxin-antitoxin system DarTG catalyzes reversible ADP-ribosylation of DNA. Mol. Cell 2016, 64, 1109–1116. Pons, L.; Vilain, C.; Volteau, M.; Picaut, P. Safety and pharmacodynamics of a novel recombinant botulinum toxin E (rBoNT-E): Results of a phase 1 study in healthy male subjects compared with abobotulinumtoxinA (Dysport®). J. Neurol. Sci. 2019, 407, 116516.
- Saunders, S.E.; Bartz, J.C.; Vercauteren, K.C.; Bartelt-Hunt, S.L. Enzymatic digestion of chronic wasting disease prions bound to soil. Environ. Sci. Technol. 2010, 44, 4129–4135. Fredericks, L.R.; Lee, M.D.; Crabtree, A.M.; Boyer, J.M.; Kizer, E.A.; Taggart, N.T.; Roslund, C.R.; Hunter, S.S.; Kennedy, C.B.; Willmore, C.G.; et al. The species-specific acquisition and diversification of a K1-like family of killer toxins in budding yeasts of the Saccharomycotina. PLoS Genet. 2021, 17, e1009341.
- Pilon, J.L.; Nash, P.B.; Arver, T.; Hoglund, D.; VerCauteren, K.C. Feasibility of infectious prion digestion using mild conditions and commercial subtilisin. J. Virol. Methods 2009, 161, 168–172. Giesselmann, E.; Becker, B.; Schmitt, M.J. Production of fluorescent and cytotoxic K28 killer toxin variants through high cell density fermentation of recombinant Pichia pastoris. Microb. Cell Fact. 2017, 16, 228.
- Dabbagh, F.; Negahdaripour, M.; Berenjian, A.; Behfar, A.; Mohammadi, F.; Zamani, M.; Irajie, C.; Ghasemi, Y. Nattokinase: Production and application. Appl. Microbiol. Biotechnol. 2014, 98, 9199–9206. Gier, S.; Schmitt, M.J.; Breinig, F. Expression of K1 toxin derivatives in Saccharomyces cerevisiae mimics treatment with exogenous toxin and provides a useful tool for elucidating K1 mechanisms of action and immunity. Toxins 2017, 9, 345.
- Hassan, M.A.; Abol-Fotouh, D.; Omer, A.M.; Tamer, T.M.; Abbas, E. Comprehensive insights into microbial keratinases and their implication in various biotechnological and industrial sectors: A review. Int. J. Biol. Macromol. 2020, 154, 567–583. Carboni, G.; Fancello, F.; Zara, G.; Zara, S.; Ruiu, L.; Marova, I.; Pinnac, G.; Budroni, M.; Mannazzu, I. Production of a lyophilized ready-to-use yeast killer toxin with possible applications in the wine and food industries. Int. J. Food Microbiol. 2020, 335, 108883.
- Chessa, R.; Landolfo, S.; Ciani, M.; Budroni, M.; Zara, S.; Ustun, M.; Cakar, Z.P.; Mannazzu, I. Biotechnological exploitation of Tetrapisisporaphaffii killer toxin: Heterologous production in Komagataellaphaffii (Pichia pastoris). Appl. Microbiol. Biotechnol. 2017, 101, 2931–2942.
- Salazar, M.H.; Clement, H.; Corrales-García, L.L.; Sánchez, J.; Cleghorn, J.; Zamudio, F.; Possani, L.D.; Acosta, H.; Corzo, G. Heterologous expression of four recombinant toxins from Panamanian scorpions of the genus Tityus and Centruroides for production of antivenom. Toxicon 2022, 13, 100090.
- Timofeev, S.; Mitina, G.; Rogozhin, E.; Dolgikh, V. Expression of spider toxin in entomopathogenic fungus Lecanicilliummuscarium and selection of the strain showing efficient secretion of the recombinant protein. FEMS Microbiol. Lett. 2019, 366, fnz181.
- Esipov, R.S.; Stepanenko, V.N.; Zvereva, I.O.; Makarov, D.A.; Kostromina, M.A.; Kostromina, T.I.; Muravyova, T.I.; Miroshnikov, A.I.; Grishin, E.V. Biotechnological method for production of recombinant peptide analgesic (purotoxin-1) from Geolycosa sp. spider poison. Russ. J. Bioorganic Chem. 2018, 44, 32–40.
- Shelukhina, I.V.; Zhmak, M.N.; Lobanov, A.V.; Ivanov, I.A.; Garifulina, A.I.; Kravchenko, I.N.; Rasskazova, E.A.; Salmova, M.A.; Tukhovskaya, E.A.; Rykov, V.A.; et al. Azemiopsin, a selective peptide antagonist of muscle nicotinic acetylcholine receptor: Preclinical evaluation as a local muscle relaxant. Toxins 2018, 10, 34.
- Babenko, V.V.; Ziganshin, R.H.; Weise, C.; Dyachenko, I.; Shaykhutdinova, E.; Murashev, A.N.; Zhmak, M.; Starkov, V.; Hoang, A.N.; Tsetlin, V.; et al. Novel bradykinin-potentiating peptides and three-finger toxins from viper venom: Combined NGS venom gland transcriptomics and quantitative venom proteomics of the Azemiops feae viper. Biomedicines 2020, 8, 249.
- Romero-Giraldo, L.E.; Pulido, S.; Berrío, M.A.; Flórez, M.F.; Rey-Suárez, P.; Nuñez, V.; Pereañez, J.A. Heterologous expression and immunogenic potential of the most abundant phospholipase a2 from coral snake Micrurus dumerilii to develop antivenoms. Toxins 2022, 14, 825.
- Esipov, R.S.; Makarov, D.A.; Stepanenko, V.N.; Kostromina, M.A.; Muravyova, T.I.; Andreev, Y.A.; Dyachenko, I.A.; Kozlov, S.A.; Grishin, E.V. Pilot production of the recombinant peptide toxin of Heteractis crispa as a potential analgesic by intein-mediated technology. Protein Expr. Purif. 2018, 145, 71–76.
- Tereshin, M.N.; Komyakova, A.M.; Stepanenko, V.N.; Myagkikh, I.V.; Shoshina, N.S.; Korolkova, Y.V.; Leychenko, E.V.; Kozlov, S.A. Optimized method for the recombinant production of a sea anemone’s peptide. Mendeleev Commun. 2022, 32, 745–746.
- Fellermann, M.; Stemmer, M.; Noschka, R.; Wondany, F.; Fischer, S.; Michaelis, J.; Stenger, S.; Barth, H. Clostridium botulinum C3 toxin for selective delivery of cargo into dendritic cells and macrophages. Toxins 2022, 14, 711.
- Fellermann, M.; Huchler, C.; Fechter, L.; Kolb, T.; Wondany, F.; Mayer, D.; Michaelis, J.; Stenger, S.; Mellert, K.; Möller, P.; et al. Clostridial C3 toxins enter and intoxicate human dendritic cells. Toxins 2020, 12, 563.
- Eisele, J.; Schreiner, S.; Borho, J.; Fischer, S.; Heber, S.; Endres, S.; Fellermann, M.; Wohlgemuth, L.; Huber-Lang, M.; Fois, G.; et al. The pore- forming subunit C2IIa of the binary Clostridium botulinum C2 toxin reduces the chemotactic translocation of human polymorphonuclear leukocytes. Front. Pharmacol. 2022, 13, 810611.
- El-Chami, D.; Al Haddad, M.; Abi-Habib, R.; El-Sibai, M. Recombinant anthrax lethal toxin inhibits cell motility and invasion in breast cancer cells through the dysregulation of Rho GTPases. Oncol. Lett. 2021, 21, 163.
- Rudenko, N.; Nagel, A.; Zamyatina, A.; Karatovskaya, A.; Salyamov, V.; Andreeva-Kovalevskaya, Z.; Siunov, A.; Kolesnikov, A.; Shepelyakovskaya, A.; Boziev, K.; et al. A monoclonal antibody against the C-terminal domain of Bacillus cereus hemolysin II inhibits HlyII cytolytic activity. Toxins 2020, 12, 806.
- Rudenko, N.; Siunov, A.; Zamyatina, A.; Melnik, B.; Nagel, A.; Karatovskaya, A.; Borisova, M.; Shepelyakovskaya, A.; Andreeva-Kovalevskaya, Z.; Kolesnikov, A.; et al. The C-terminal domain of Bacillus cereus hemolysin II oligomerizes by itself in the presence of cell membranes to form ion channels. Int. J. Biol. Macromol. 2022, 200, 416–427.
- Maksimov, I.V.; Blagova, D.K.; Veselova, S.V.; Sorokan, A.V.; Burkhanova, G.F.; Cherepanova, E.A.; Sarvarova, E.R.; Rumyantsev, S.D.; Alekseev, V.Y.; Khayrullin, R.M. Recombinant Bacillus subtilis 26DCryChS line with gene Btcry1Ia encoding Cry1Ia toxin from Bacillus thuringiensis promotes integrated wheat defense against pathogen Stagonospora nodorum Berk. and greenbug Schizaphis graminum Rond. Biol. Control 2020, 144, 104242.
- Zakharzhevskaya, N.B.; Tsvetkov, V.B.; Vanyushkina, A.A.; Varizhuk, A.M.; Rakitina, D.V.; Podgorsky, V.V.; Vishnyakov, I.E.; Kharlampieva, D.D.; Manuvera, V.A.; Lisitsyn, F.V.; et al. Interaction of bacteroides fragilis toxin with outer membrane vesicles reveals new mechanism of its secretion and delivery. Front. Cell. Infect. Microbiol. 2017, 7, 2.
- Voltà-Durán, E.; Sánchez, J.M.; Parladé, E.; Serna, N.; Vazquez, E.; Unzueta, U.; Villaverde, A. The Diphtheria toxin translocation domain impairs receptor selectivity in cancer cell-targeted protein nanoparticles. Pharmaceutics 2022, 14, 2644.
- Manoilov, K.Y.; Labyntsev, A.J.; Korotkevych, N.V.; Maksymovych, I.S.; Kolybo, D.V.; Komisarenko, S.V. Particular features of diphtheria toxin internalization by resistant and sensitive mammalian cells. Cytol. Genet. 2018, 52, 353–359.
- Chalenko, Y.; Sobyanin, K.; Sysolyatina, E.; Midiber, K.; Kalinin, E.; Lavrikova, A.; Mikhaleva, L.; Ermolaeva, S. Hepatoprotective Activity of InlB321/15, the HGFR Ligand of Bacterial Origin, in CCI4-Induced Acute Liver Injury Mice. Biomedicines 2019, 7, 29.
- Abramov, V.M.; Kosarev, I.V.; Motin, V.L.; Khlebnikov, V.S.; Vasilenko, R.N.; Sakulin, V.K.; Machulin, A.V.; Uversky, V.N.; Karlyshev, A.V. Binding of LcrV protein from Yersinia pestis to human T-cells induces apoptosis, which is completely blocked by specific antibodies. Int. J. Biol. Macromol. 2019, 122, 1062–1070.
- Ovchinnikov, S.V.; Bikmetov, D.; Livenskyi, A.; Serebryakova, M.; Wilcox, B.; Mangano, K.; Shiriaev, D.I.; Osterman, I.A.; Sergiev, P.V.; Borukhov, S.; et al. Mechanism of translation inhibition by type II GNAT toxin AtaT2. Nucleic Acids Res. 2020, 48, 8617–8625.
- Carboni, G.; Marova, I.; Zara, G.; Zara, S.; Budroni, M.; Mannazzu, I. Evaluation of recombinant Kpkt cytotoxicity on HaCaT cells: Further steps towards the biotechnological exploitation yeast killer toxins. Foods 2021, 10, 556.
- Gandomkari, M.S.; Ayat, H.; Ahadi, A.M. Recombinantly expressed MeICT, a new toxin from Mesobuthuseupeus scorpion, inhibits glioma cell proliferation and downregulates Annexin A2 and FOXM1 genes. Biotechnol. Lett. 2022, 44, 703–712.
- Kuzmenkov, A.I.; Nekrasova, O.V.; Peigneur, S.; Tabakmakher, V.M.; Gigolaev, A.M.; Fradkov, A.F.; Kudryashova, K.S.; Chugunov, A.O.; Efremov, A.G.; Tytgat, J.; et al. KV1. 2 channel-specific blocker from Mesobuthuseupeus scorpion venom: Structural basis of selectivity. Neuropharmacology 2018, 143, 228–238.
- Gigolaev, A.M.; Kuzmenkov, A.I.; Peigneur, S.; Tabakmakher, V.M.; Pinheiro-Junior, E.L.; Chugunov, A.O.; Efremov, R.G.; Tytgat, J.; Vassilevski, A.A. Tuning scorpion toxin selectivity: Switching from KV1.1 to KV1.3. Front. Pharmacol. 2020, 11, 1010.
- Korolkova, Y.; Maleeva, E.; Mikov, A.; Lobas, A.; Solovyeva, E.; Gorshkov, M.; Andreev, Y.; Peigneur, S.; Tytgat, J.; Kornilov, F.; et al. New insectotoxin from Tibellus oblongus spider venom presents novel adaptation of ICK fold. Toxins 2021, 13, 29.
- Terpinskaya, T.I.; Osipov, A.V.; Kryukova, E.V.; Kudryavtsev, D.S.; Kopylova, N.V.; Yanchanka, T.L.; Palukoshka, A.F.; Gondarenko, E.A.; Zhmak, M.N.; Tsetlin, V.I.; et al. α -Conotoxins and α-Cobratoxin promote, while lipoxygenase and cyclooxygenase inhibitors suppress the proliferation of glioma C6 cells. Mar. Drugs 2021, 19, 118.
- Makarova, Y.V.; Kryukova, E.V.; Shelukhina, I.V.; Lebedev, D.S.; Andreeva, T.V.; Ryazantsev, D.Y.; Balandin, S.V.; Ovchinnikova, T.V.; Tsetlin, V.I.; Utkin, Y.N. The first recombinant viper three-finger toxins: Inhibition of muscle and neuronal nicotinic acetylcholine receptors. Dokl. Biochem. Biophys. 2018, 479, 127–130.
- Son, L.; Kryukova, E.; Ziganshin, R.; Andreeva, T.; Kudryavtsev, D.; Kasheverov, I.; Tsetlin, V.; Utkin, Y. Novel three-finger neurotoxins from Naja melanoleuca cobra venom interact with GABAA and nicotinic acetylcholine receptors. Toxins 2021, 13, 164.
- Kvetkina, A.; Malyarenko, O.; Pavlenko, A.; Dyshlovoy, S.; von Amsberg, G.; Ermakova, S.; Leychenko, E. Sea anemone Heteractis crispa actinoporin demonstrates in vitro anticancer activities and prevents HT-29 colorectal cancer cell migration. Molecules 2020, 25, 5979.
- Godakova, S.A.; Noskov, A.N.; Vinogradova, I.D.; Ugriumova, G.A.; Solovyev, A.I.; Esmagambetov, I.B.; Tukhvatulin, A.I.; Logunov, D.Y.; Naroditsky, B.S.; Shcheblyakov, D.V.; et al. Camelid VHHs fused to human Fc fragments provide long term protection against botulinum neurotoxin a in mice. Toxins 2019, 11, 464.
- Rodrigues, R.R.; Ferreira, M.R.A.; Donassolo, R.A.; Alves, M.L.F.; Motta, J.F.; Moreira, C., Jr.; Salvarani, F.M.; Moreira, A.N.; Conceição, F.R. Evaluation of the expression and immunogenicity of four versions of recombinant Clostridium perfringens beta toxin designed by bioinformatics tools. Anaerobe 2021, 69, 102326.
- Ferreira, D.V.; dos Santos, F.D.; da Cunha, C.E.P.; Moreira, C., Jr.; Donassolo, R.A.; Magalhães, C.G.; Belo Reis, A.S.; Oliveira, C.M.C.; Barbosa, J.D.; Leite, F.P.L.; et al. Immunogenicity of Clostridium perfringens epsilon toxin recombinant bacterin in rabbit and ruminants. Vaccine 2018, 36, 7589–7592.
- Karpov, D.S.; Goncharenko, A.V.; Usachev, E.V.; Vasina, D.V.; Divisenko, E.V.; Chalenko, Y.M.; Pochtovyi, A.A.; Ovchinnikov, R.S.; Makarov, V.V.; Yudin, S.M.; et al. A Strategy for the Rapid Development of a Safe Vibrio cholerae Candidate Vaccine Strain. Int. J. Mol. Sci. 2021, 22, 11657.
- Alomran, N.; Blundell, P.; Alsolaiss, J.; Crittenden, E.; Ainsworth, S.; Dawson, C.A.; Edge, R.J.; Hall, S.R.; Harrison, R.A.; Wilkinson, M.C.; et al. Exploring the utility of recombinant snake venom serine protease toxins as immunogens for generating experimental snakebite antivenoms. Toxins 2022, 14, 443.
- Alomran, N.; Chinnappan, R.; Alsolaiss, J.; Casewell, N.R.; Zourob, M. Exploring the utility of ssDNA aptamers directed against snake venom toxins as new therapeutics for snakebite envenoming. Toxins 2022, 14, 469.
- Neuschäfer-Rube, F.; Pathe-Neuschäfer-Rube, A.; Püschel, G.P. Discrimination of the activity of low-affinity wild-type and high-affinity mutant recombinant BoNT/B by a SIMA cell-based reporter release assay. Toxins 2022, 14, 65.
- Martin, T.; Möglich, A.; Felix, I.; Förtsch, C.; Rittlinger, A.; Palmer, A.; Denk, S.; Schneider, J.; Notbohm, L.; Vogel, M.; et al. Rho-inhibiting C2IN-C3 fusion toxin inhibits chemotactic recruitment of human monocytes ex vivo and in mice in vivo. Arch. Toxicol. 2018, 92, 323–336.
- Hashemi Yeganeh, H.; Heiat, M.; Kieliszek, M.; Alavian, S.M.; Rezaie, E. DT389-YP7, a recombinant immunotoxin against glypican-3 that inhibits hepatocellular cancer cells: An in vitro study. Toxins 2021, 13, 749.
- Cheung, L.S.; Fu, J.; Kumar, P.; Kumar, A.; Urbanowski, M.E.; Ihms, E.A.; Parveen, S.; Bullen, C.K.; Patrick, G.J.; Harrison, R.; et al. Second-generation IL-2 receptor-targeted diphtheria fusion toxin exhibits antitumor activity and synergy with anti–PD-1 in melanoma. Proc. Natl. Acad. Sci. USA 2019, 116, 3100–3105.
- Schmohl, J.U.; Todhunter, D.; Taras, E.; Bachanova, V.; Vallera, D.A. Development of a deimmunized bispecific immunotoxin dDT2219 against B-cell malignancies. Toxins 2018, 10, 32.
- Ryabchevskaya, E.M.; Evtushenko, A.; Granovskiy, D.L.; Ivanov, P.A.; Atabekov, J.G.; Kondakova, O.A.; Nikitin, N.A.; Karpova, O.V. Two approaches for the stabilization of Bacillus anthracis recombinant protective antigen. Hum. Vaccines Immunother. 2021, 17, 560–565.
- Ryabchevskaya, E.M.; Granovskiy, D.L.; Evtushenko, E.A.; Ivanov, P.A.; Kondakova, O.A.; Nikitin, N.A.; Karpova, O.V. Designing stable Bacillus anthracis antigens with a view to recombinant anthrax vaccine development. Pharmaceutics 2022, 14, 806.
- Evtushenko, E.A.; Kondakova, O.A.; Arkhipenko, M.V.; Kravchenko, T.B.; Bakhteeva, I.V.; Timofeev, V.S.; Nikitin, N.A.; Karpova, O.V. New formulation of a recombinant anthrax vaccine stabilised with structurally modified plant viruses. Front. Microbiol. 2022, 13, 1003969.
- Shulcheva, I.; Shchannikova, M.; Melnik, B.; Fursova, K.; Semushina, S.; Zamyatina, A.; Oleinikov, V.; Brovko, F. The zinc ions stabilize the three-dimensional structure and are required for the binding of staphylococcal enterotoxin-like protein P (SEIP) with MHC-II receptors. Protein Expr. Purif. 2022, 197, 106098.
- Gholami, N.; Cohan, R.A.; Razavi, A.; Bigdeli, R.; Dashbolaghi, A.; Asgary, V. Cytotoxic and apoptotic properties of a novel nano-toxin formulation based on biologically synthesized silver nanoparticle loaded with recombinant truncated Pseudomonas exotoxin A. J. Cell. Physiol. 2020, 235, 3711–3720.
- Royal, J.M.; Reeves, M.A.; Matoba, N. Repeated oral administration of a KDEL-tagged recombinant cholera toxin B subunit effectively mitigates dss colitis despite a robust immunogenic response. Toxins 2019, 11, 678.
- Nekrasova, O.V.; Primak, A.L.; Ignatova, A.A.; Novoseletsky, V.N.; Geras’kina, O.V.; Kudryashova, K.S.; Yakimov, S.A.; Kirpichnikov, M.P.; Arseniev, A.S.; Feofanov, A.V. N-terminal tagging with GFP enhances selectivity of agitoxin 2 to Kv1.3-channel binding site. Toxins 2020, 12, 802.
- Calabria, P.A.; Shimokawa-Falcão, L.H.A.; Colombini, M.; Moura-da-Silva, A.M.; Barbaro, K.C.; Faquim-Mauro, E.L.; Magalhaes, G.S. Design and production of a recombinant hybrid toxin to raise protective antibodies against Loxosceles spider venom. Toxins 2019, 11, 108.
- Logashina, Y.A.; Lubova, K.I.; Maleeva, E.E.; Palikov, V.A.; Palikova, Y.A.; Dyachenko, I.A.; Andreev, Y.A. Analysis of structural determinants of peptide MS 9a-1 essential for potentiating of TRPA1 channel. Mar. Drugs 2022, 20, 465.
- Mannazzu, I.; Domizio, P.; Carboni, G.; Zara, S.; Zara, G.; Comitini, F.; Budroni, M.; Ciani, M. Yeast killer toxins: From ecological significance to application. Crit. Rev. Biotechnol. 2019, 39, 603–617.
- Giovati, L.; Ciociola, T.; De Simone, T.; Conti, S.; Magliani, W. Wickerhamomyces yeast killer toxins’ medical applications. Toxins 2021, 13, 655.
- Leychenko, E.; Isaeva, M.; Tkacheva, E.; Zelepuga, E.; Kvetkina, A.; Guzev, K.; Monastyrnaya, M.; Kozlovskaya, E. Multigene family of pore-forming toxins from sea anemone Heteractis crispa. Mar. Drugs 2018, 16, 183.
- Ma, J.; Zhang, J.; Yan, R. Recombinant mammalian prions: The “correctly” misfolded prion protein conformers. Viruses 2022, 14, 1940.
- Imamura, M.; Tabeta, N.; Iwamaru, Y.; Takatsuki, H.; Mori, T.; Atarashi, R. Spontaneous generation of distinct prion variants with recombinant prion protein from a baculovirus-insect cell expression system. Biochem. Biophys. Res. Commun. 2022, 613, 67–72.
- Abskharon, R.; Wang, F.; Wohlkonig, A.; Ruan, J.; Soror, S.; Giachin, G.; Pardon, E.; Zou, W.; Legname, G.; Ma, J.; et al. Structural evidence for the critical role of the prion protein hydrophobic region in forming an infectious prion. PLoS Pathog. 2019, 15, e1008139.
- Abdelaziz, D.H.; Thapa, S.; Brandon, J.; Maybee, J.; Vankuppeveld, L.; McCorkell, R.; Schätzl, H.M. Recombinant prion protein vaccination of transgenic elk PrP mice and reindeer overcomes self-tolerance and protects mice against chronic wasting disease. J. Biol. Chem. 2018, 293, 19812–19822.
- Hwang, S.; Tatum, T.; Lebepe-Mazur, S.; Nicholson, E.M. Preparation of lyophilized recombinant prion protein for TSE diagnosis by RT-QuIC. BMC Res. Notes 2018, 11, 895.
- Kovachev, P.S.; Gomes, M.P.; Cordeiro, Y.; Ferreira, N.C.; Valadão, L.P.F.; Ascari, L.M.; Rangel, L.P.; Silva, J.L.; Sanyal, S. RNA modulates aggregation of the recombinant mammalian prion protein by direct interaction. Sci. Rep. 2019, 9, 12406.
- Benilova, I.; Reilly, M.; Terry, C.; Wenborn, A.; Schmidt, C.; Marinho, A.T.; Risse, E.; Al-Doujaily, H.; WigginsDeOliveira, M.; Sandberg, M.K.; et al. Highly infectious prions are not directly neurotoxic. Proc. Natl. Acad. Sci. USA 2020, 117, 23815.
- Fernández-Borges, N.; Di Bari, M.A.; Eraña, H.; Sánchez-Martín, M.; Pirisinu, L.; Parra, B.; Elezgarai, S.R.; Vanni, I.; López-Moreno, R.; Vaccari, G.; et al. Cofactors influence the biological properties of infectious recombinant prions. Acta. Neuropathol. 2018, 135, 179–199.
- Jack, K.; Jackson, G.S.; Bieschke, J. Essential components of synthetic infectious prion formation de novo. Biomolecules 2022, 12, 1694.
- Hassan, M.A.; Abol-Fotouh, D.; Omer, A.M.; Tamer, T.M.; Abbas, E. Comprehensive insights into microbial keratinases and their implication in various biotechnological and industrial sectors: A review. Int. J. Biol. Macromol. 2020, 154, 567–583.
More
