Autophagy–the lysosomal degradation of cytoplasm–plays a central role in cellular homeostasis and protects cells from potentially harmful agents that may accumulate in the cytoplasm, including pathogens, protein aggregates, and dysfunctional organelles. This process is initiated by the formation of a phagophore membrane, which wraps around a portion of cytoplasm or cargo and closes to form a double-membrane autophagosome. Upon the fusion of the autophagosome with a lysosome, the sequestered material is degraded by lysosomal hydrolases in the resulting autolysosome. Several alternative membrane sources of autophagosomes have been proposed, including the plasma membrane, endosomes, mitochondria, endoplasmic reticulum, lipid droplets, hybrid organelles, and de novo synthesis.
- autophagy
- autophagosome
- endosome
1. Introduction
2. The Preautophagosomal Structure as Autophagosome Generator in Budding Yeast
Light and electron microscopic studies of budding yeast exposed to nitrogen starvation have revealed a single preautophagosomal structure (PAS, also called a phagophore assembly site) located close to the vacuole, which is the yeast equivalent of lysosomes [9]. The PAS organizes a number of proteins involved in phagophore initiation and expansion (Figure 1). First, the protein kinase Atg1 and its complex partners Atg13, Atg17, Atg29, and Atg31 form the initial scaffold of the PAS, and subsequently, other Atg proteins, including a ubiquitin-like protein (Atg8), its conjugation machinery (Atg7, Atg3, Atg5-Atg12, Atg16), and a phosphatidylinositol 3-kinase (PI3K) complex (Vps15, Vps34, Atg6, Atg14) assemble onto this scaffold. Anchoring the PAS to the vacuole appears to be largely mediated by the interaction of the PAS protein Atg13 with the vacuole protein Vac8 [10].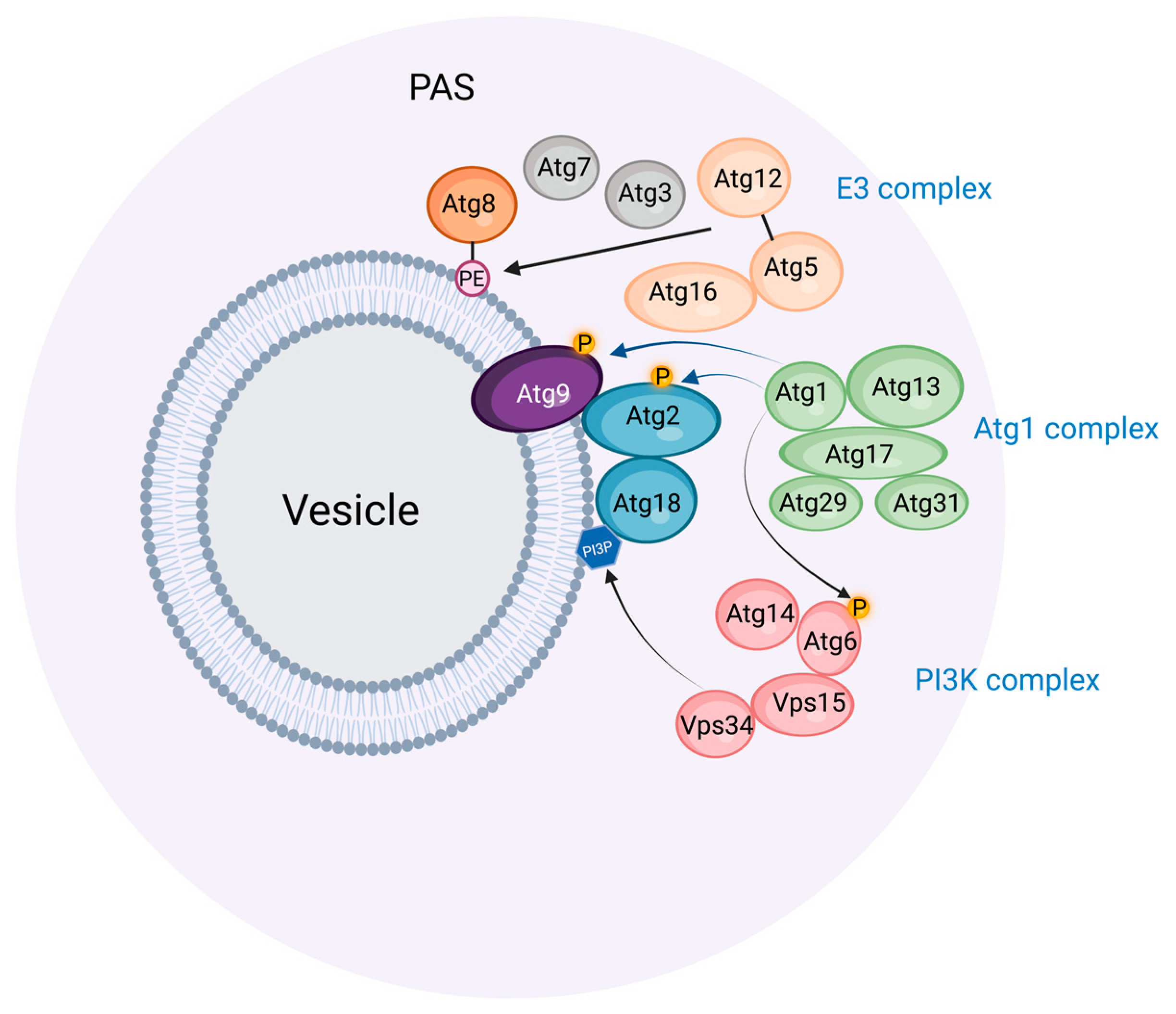
3. Similarities and Differences between Budding Yeast and Mammals in Autophagosome Biogenesis
The remarkable conservation of the autophagic machinery components between yeast and mammals indicates that the basic principles of autophagosome biogenesis have been conserved through evolution. However, whereas the PAS has a central role in autophagosome biogenesis in yeast, no direct equivalent to the PAS has so far been identified in mammalian cells. This does not necessarily mean that no such equivalent exists since discrete phase-separated PAS-like structures would be difficult to detect in the crowded cytoplasm of mammalian cells, especially if the structures are short-lived. However, it is also possible that mammalian ATG proteins could assemble on membranes in an organized pattern instead of forming liquid-like assemblies. Mammalian cells are generally much larger than yeast cells and typically have hundreds of lysosomes compared with single vacuoles in yeast cells. Perhaps owing to these differences, multiple membranes have been identified as potential sources of autophagosome membranes in mammals, and these are discussed in the following sections.4. The Endoplasmic Reticulum as Source of Phagophores
In most mammalian cells, the endoplasmic reticulum (ER) extends as a tubulocisternal network throughout the cell body. It is thus well positioned as a potential source for autophagic membranes, and there is indeed good evidence that the ER plays an important role in autophagosome biogenesis. Electron tomography shows that phagophores are often tightly associated with ER membranes, either in continuity or closely apposed [42,43][22][23]. In fact, quantitative 3D light and electron microscopy indicate that all phagophores are associated with the ER [44][24]. The PI3P-binding ER protein DFCP1/ZFYVE1 is frequently observed at sites of phagophore biogenesis during starvation. DFCP1-positive membrane structures typically have an omega shape during starvation-induced autophagy and are, therefore, referred to as omegasomes [27][25]. Even though DFCP1 is nonessential for bulk autophagy, omegasomes have been proposed to form domains of the ER that function as cradles for nascent phagophores. There is some disambiguity in the literature on whether the ER membranes are continuous with the forming phagophore or not [42,43,45][22][23][26]. Although both scenarios may be correct, a physical separation between the ER and the growing phagophore would be most consistent with what is known about phagophore assembly in the yeast and also with recent studies that involve ATG9-containing vesicles. The discoveries that ATG9 is a lipid scramblase [21,22][27][28] and ATG2 a lipid channel [12][29] have offered a plausible mechanism for how the ER can provide lipids to the growing phagophore. Single or multiple ATG9 vesicles coming from the Golgi could act as the seed for phagophore formation [46][30]. Upon docking to the ER via ATG2, lipids synthesized in the ER could be transferred to the ATG9-containing phagophore seed, thereby promoting its expansion. ATG9, which forms a complex with ATG2 [47][31], would function to equilibrate the channeled lipids into the inner phagophore membrane bilayer in order to allow membrane expansion. On the ER side, two other lipid scramblases, VMP1 and TMEM41B, would have an equivalent role in equilibrating levels of newly synthesized lipids over the ER membrane. ATG2 attaches to the phagophore membrane via the binding of its C-terminus to ATG9 and the Atg18 homolog WIPI4, whereas its N-terminus interacts with VMP1 and TMEM41B in the ER membrane [48][32] (Figure 2). In addition to ATG2, the structurally related VPS13 proteins have also been implicated in autophagy [49,50[33][34][35],51], indicating a role for multiple lipid channels in phagophore expansion.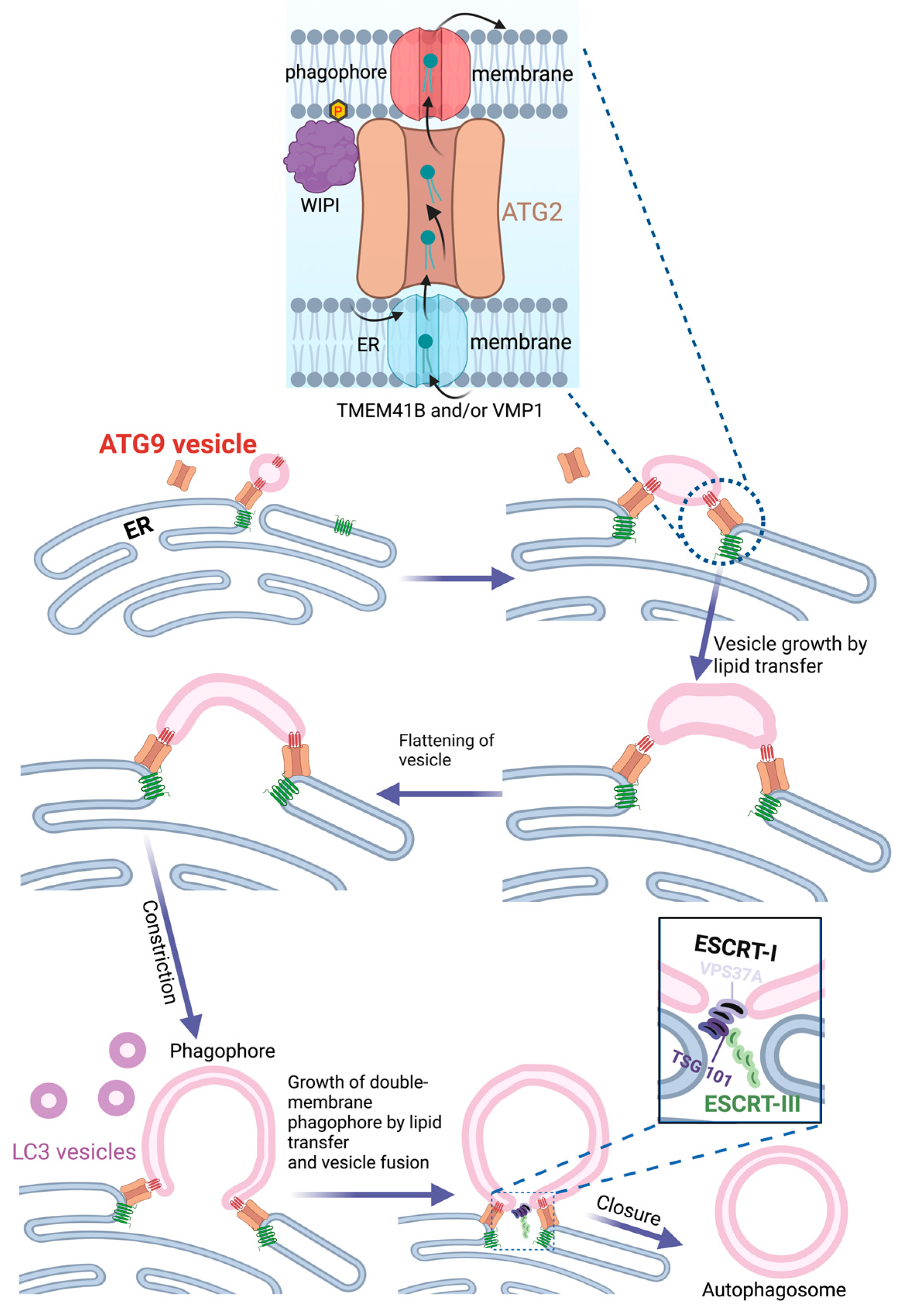
5. COPII Vesicles and ER Exit Sites in Phagophore Biogenesis
ER exit sites are specialized regions of the ER where COPII transport vesicles are generated. These vesicles, which are best known for their role in ER-Golgi transport [54][38], are interesting in the context of autophagy since COPII subunits are essential for starvation-induced autophagy in budding yeast [55][39]. In this organism, autophagosomes are formed very close to ER exit sites, and the function of these sites is required for PAS formation [56][40]. Under starvation conditions, the secretory pathway is largely shut down, and COPII vesicles, instead of fusing with the Golgi, are diverted to the PAS. If autophagy is blocked, the COPII vesicles accumulate at the PAS via binding to Sec23. The small GTPase Ypt1 (homolog of mammalian RAB1) is activated by its guanine nucleotide exchange factor complex, TRAPPIII, and, together with Atg17, these proteins serve to tether Atg9 vesicles to COPII vesicles [57][41], thereby providing potential seeds for the phagophore. Although most information on COPII and ER exit sites in autophagy has been obtained through studies of budding yeast, there is evidence that similar mechanisms could operate in mammalian cells. In particular, a mammalian TRAPPIII complex has been found to function as a GEF for RAB1 and to regulate autophagy, though it appears to regulate the trafficking of ATG9 vesicles from recycling endosomes to the Golgi instead of tethering ER-derived vesicles to the forming phagophore [58,59][42][43]. In addition to its proposed role in vesicle tethering, RAB1 has been shown to interact with the autophagy-specific PI3K complex [60][44], and yeast Ypt1 interacts with the autophagy-initiating kinase Atg1 at the PAS [61][45]. This raises the possibility that RAB1, trafficked on COPII vesicles, might play a role in Atg1/ULK1 and PI3K recruitment during the initiation of autophagy.6. Mitochondria as Source of Phagophore Membranes
As major sites for ATP production and lipid metabolism, mitochondria are central to cellular metabolism. Mitochondrial membranes, which consist of two layers, are highly dynamic and undergo fusion, fission, and budding reactions that are controlled by metabolic cues. An interesting aspect of the outer mitochondrial membranes is that they have been implicated as membrane sources for starvation-induced autophagosomes [62][46]. Evidence for this includes the detection of ATG5 on mitochondria during starvation, the translocation of a targeting sequence derived from a mitochondrial outer membrane protein to autophagosomes, and the delivery of fluorescently tagged lipids from mitochondria to autophagosomes. Moreover, the depletion of Mitofusin2, which not only mediates mitochondrial fusion but also forms contact sites between the mitochondria and ER, strongly inhibits starvation-induced autophagy. Analyzing the involvement of mitochondria in autophagosome biogenesis is complicated by the fact that damaged mitochondria, which might occur during starvation, are themselves targeted by autophagy in a process known as mitophagy [63][47]. However, under starvation conditions that are sufficient to induce translocation of a mitochondrial outer membrane reporter to autophagosomes, no mitochondrial matrix proteins could be detected in the autophagosomes, arguing that the detected contribution of mitochondrial membranes to autophagosomes is not due to mitophagy [62][46]. How well suited is the mitochondrial outer membrane as a source of phagophore membranes? This membrane contains abundant mitochondrial outer membrane proteins, and these are generally excluded from autophagosomes, suggesting that there must exist some gating mechanism that prevents most mitochondrial proteins from entering phagophores [62][46]. On the other hand, it is interesting that PE, which is conjugated to Atg8 family proteins to mediate their membrane anchoring and, thereby, their function in phagophore expansion, is synthesized in both mitochondria and the ER [64][48]. Mitochondrial outer membranes could, therefore, serve as a source of PE.7. Contact Sites between ER and Mitochondria as Source of Phagophores
A model that unifies the notions of phagophore biogenesis from ER and mitochondria, respectively, has emerged through the observation that the membrane-targeting component of the autophagic PI3K complex, ATG14, is recruited to contact sites between ER and mitochondria upon starvation, by binding the ER-resident SNARE protein Syntaxin-17 [65][49]. The depletion of PACS-2, a protein important for the formation of ER-mitochondria contacts [66][50], strongly inhibits the recruitment of ATG14 and DFCP1 to ER-mitochondria contact sites during starvation and also decreases the PE conjugation of the Atg8 family protein LC3, indicating that ER-mitochondria contacts are involved in phagophore biogenesis [65][49]. The finding that ATG9-containing vesicles are mobilized to these sites is consistent with this idea [67][51]. It still remains to be understood how ATG14 and DFCP1 are targeted specifically to ER-mitochondria contact sites by Syntaxin-17 during starvation and exactly how the phagophore forms at the contact sites.References
- Nakatogawa, H.; Suzuki, K.; Kamada, Y.; Ohsumi, Y. Dynamics and diversity in autophagy mechanisms: Lessons from yeast. Nat. Rev. Mol. Cell Biol. 2009, 10, 458–467.
- Dikic, I.; Elazar, Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364.
- Seglen, P.O.; Gordon, P.B.; Holen, I. Non-selective autophagy. Semin. Cell Biol. 1990, 1, 441–448.
- Takeshige, K.; Baba, M.; Tsuboi, S.; Noda, T.; Ohsumi, Y. Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J. Cell Biol. 1992, 119, 301–311.
- Gudmundsson, S.R.; Kallio, K.A.; Vihinen, H.; Jokitalo, E.; Ktistakis, N.; Eskelinen, E.-L. Morphology of Phagophore Precursors by Correlative Light-Electron Microscopy. Cells 2022, 11, 3080.
- Berg, T.O.; Fengsrud, M.; Strømhaug, P.E.; Berg, T.; Seglen, P.O. Isolation and characterization of rat liver amphisomes—Evidence for fusion of autophagosomes with both early and late endosomes. J. Biol. Chem. 1998, 273, 21883–21892.
- Yu, L.; McPhee, C.K.; Zheng, L.; Mardones, G.A.; Rong, Y.; Peng, J.; Mi, N.; Zhao, Y.; Liu, Z.; Wan, F.; et al. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature 2010, 465, 942–946.
- Melia, T.J.; Lystad, A.H.; Simonsen, A. Autophagosome biogenesis: From membrane growth to closure. J. Cell Biol. 2020, 219, e202002085.
- Suzuki, K.; Ohsumi, Y. Current knowledge of the pre-autophagosomal structure (PAS). FEBS Lett. 2010, 584, 1280–1286.
- Hollenstein, D.; Gómez-Sánchez, R.; Ciftci, A.M.A.; Kriegenburg, F.; Mari, M.; Torggler, R.; Licheva, M.; Reggiori, F.; Kraft, C. Vac8 spatially confines autophagosome formation at the vacuole. J. Cell Sci. 2019, 132, jcs235002.
- Fujioka, Y.; Alam, J.M.; Noshiro, D.; Mouri, K.; Ando, T.; Okada, Y.; May, A.I.; Knorr, R.L.; Suzuki, K.; Ohsumi, Y.; et al. Phase separation organizes the site of autophagosome formation. Nature 2020, 578, 301–305.
- Yamamoto, H.; Fujioka, Y.; Suzuki, S.W.; Noshiro, D.; Suzuki, H.; Kondo-Kakuta, C.; Kimura, Y.; Hirano, H.; Ando, T.; Noda, N.N.; et al. The Intrinsically Disordered Protein Atg13 Mediates Supramolecular Assembly of Autophagy Initiation Complexes. Dev. Cell 2016, 38, 86–99.
- Mizushima, N.; Yoshimori, T.; Ohsumi, Y. The Role of Atg Proteins in Autophagosome Formation. Annu. Rev. Cell Dev. Biol. 2011, 27, 107–132.
- Kihara, A.; Noda, T.; Ishihara, N.; Ohsumi, Y. Two Distinct Vps34 Phosphatidylinositol 3–Kinase Complexes Function in Autophagy and Carboxypeptidase Y Sorting inSaccharomyces cerevisiae. J. Cell Biol. 2001, 152, 519–530.
- Kotani, T.; Kirisako, H.; Koizumi, M.; Ohsumi, Y.; Nakatogawa, H. The Atg2-Atg18 complex tethers pre-autophagosomal membranes to the endoplasmic reticulum for autophagosome formation. Proc. Natl. Acad. Sci. USA 2018, 115, 10363–10368.
- Obara, K.; Ohsumi, Y. Dynamics and function of PtdIns(3)P in autophagy. Autophagy 2008, 4, 952–954.
- Ramirez, S.C.; Gómez-Sánchez, R.; Verlhac, P.; Hardenberg, R.; Margheritis, E.; Cosentino, K.; Reggiori, F.; Ungermann, C. Atg9 interactions via its transmembrane domains are required for phagophore expansion during autophagy. Autophagy 2022, 1–20.
- Cebollero, E.; Van Der Vaart, A.; Reggiori, F. Understanding phosphatidylinositol-3-phosphate dynamics during autophagosome biogenesis. Autophagy 2012, 8, 1868–1870.
- Noda, N.N.; Inagaki, F. Mechanisms of Autophagy. Annu. Rev. Biophys. 2015, 44, 101–122.
- Nakatogawa, H.; Ichimura, Y.; Ohsumi, Y. Atg8, a Ubiquitin-like Protein Required for Autophagosome Formation, Mediates Membrane Tethering and Hemifusion. Cell 2007, 130, 165–178.
- Maruyama, T.; Alam, J.M.; Fukuda, T.; Kageyama, S.; Kirisako, H.; Ishii, Y.; Shimada, I.; Ohsumi, Y.; Komatsu, M.; Kanki, T.; et al. Membrane perturbation by lipidated Atg8 underlies autophagosome biogenesis. Nat. Struct. Mol. Biol. 2021, 28, 583–593.
- Hayashi-Nishino, M.; Fujita, N.; Noda, T.; Yamaguchi, A.; Yoshimori, T.; Yamamoto, A. A subdomain of the endoplasmic reticulum forms a cradle for autophagosome formation. Nat. Cell Biol. 2009, 11, 1433–1437.
- Ylä-Anttila, P.; Vihinen, H.; Jokitalo, E.; Eskelinen, E.-L. 3D tomography reveals connections between the phagophore and endoplasmic reticulum. Autophagy 2009, 5, 1180–1185.
- Takahashi, S.; Saito, C.; Koyama-Honda, I.; Mizushima, N. Quantitative 3D correlative light and electron microscopy of organelle association during autophagy. Cell Struct. Funct. 2022, 47, 89–99.
- Axe, E.L.; Walker, S.A.; Manifava, M.; Chandra, P.; Roderick, H.L.; Habermann, A.; Griffiths, G.; Ktistakis, N.T. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J. Cell Biol. 2008, 182, 685–701.
- Kishi-Itakura, C.; Koyama-Honda, I.; Itakura, E.; Mizushima, N. Ultrastructural analysis of autophagosome organization using mammalian autophagy-deficient cells. J. Cell Sci. 2014, 127, 4089–4102.
- Matoba, K.; Kotani, T.; Tsutsumi, A.; Tsuji, T.; Mori, T.; Noshiro, D.; Sugita, Y.; Nomura, N.; Iwata, S.; Ohsumi, Y.; et al. Atg9 is a lipid scramblase that mediates autophagosomal membrane expansion. Nat. Struct. Mol. Biol. 2020, 27, 1185–1193.
- Maeda, S.; Yamamoto, H.; Kinch, L.N.; Garza, C.M.; Takahashi, S.; Otomo, C.; Grishin, N.V.; Forli, S.; Mizushima, N.; Otomo, T. Structure, lipid scrambling activity and role in autophagosome formation of ATG9A. Nat. Struct. Mol. Biol. 2020, 27, 1194–1201.
- Valverde, D.P.; Yu, S.; Boggavarapu, V.; Kumar, N.; Lees, J.A.; Walz, T.; Reinisch, K.M.; Melia, T.J. ATG2 transports lipids to promote autophagosome biogenesis. J. Cell Biol. 2019, 218, 1787–1798.
- Ghanbarpour, A.; Valverde, D.P.; Melia, T.J.; Reinisch, K.M. A model for a partnership of lipid transfer proteins and scramblases in membrane expansion and organelle biogenesis. Proc. Natl. Acad. Sci. USA 2021, 118, e2101562118.
- van Vliet, A.R.; Chiduza, G.N.; Maslen, S.L.; Pye, V.E.; Joshi, D.; De Tito, S.; Jefferies, H.B.; Christodoulou, E.; Roustan, C.; Punch, E.; et al. ATG9A and ATG2A form a heteromeric complex essential for autophagosome formation. Mol. Cell 2022, 82, 4324–4339.e8.
- Melia, T.J.; Reinisch, K.M. A possible role for VPS13-family proteins in bulk lipid transfer, membrane expansion and organelle biogenesis. J. Cell Sci. 2022, 135, jcs259357.
- Muñoz-Braceras, S.; Calvo, R.; Escalante, R. TipC and the chorea-acanthocytosis protein VPS13A regulate autophagy in Dictyostelium and human HeLa cells. Autophagy 2015, 11, 918–927.
- Kumar, N.; Leonzino, M.; Hancock-Cerutti, W.; Horenkamp, F.A.; Li, P.; Lees, J.A.; Wheeler, H.; Reinisch, K.M.; De Camilli, P. VPS13A and VPS13C are lipid transport proteins differentially localized at ER contact sites. J. Cell Biol. 2018, 217, 3625–3639.
- Chen, S.; Mari, M.; Parashar, S.; Liu, D.; Cui, Y.; Reggiori, F.; Novick, P.J.; Ferro-Novick, S. Vps13 is required for the packaging of the ER into autophagosomes during ER-phagy. Proc. Natl. Acad. Sci. USA 2020, 117, 18530–18539.
- Fengsrud, M.; Erichsen, E.S.; Berg, T.O.; Raiborg, C.; Seglen, P.O. Ultrastructural characterization of the delimiting membranes of isolated autophagosomes and amphisomes by freeze-fracture electron microscopy. Eur. J. Cell Biol. 2000, 79, 871–882.
- Ravussin, A.; Brech, A.; Tooze, S.A.; Stenmark, H. The phosphatidylinositol 3-phosphate-binding protein SNX4 controls ATG9A recycling and autophagy. J. Cell Sci. 2021, 134, jcs250670.
- Antonny, B.; Schekman, R. ER export: Public transportation by the COPII coach. Curr. Opin. Cell Biol. 2001, 13, 438–443.
- Ishihara, N.; Hamasaki, M.; Yokota, S.; Suzuki, K.; Kamada, Y.; Kihara, A.; Yoshimori, T.; Noda, T.; Ohsumi, Y. Autophagosome requires specific early Sec proteins for its formation and NSF/SNARE for vacuolar fusion. Mol. Biol. Cell 2001, 12, 3690–3702.
- Graef, M.; Friedman, J.; Graham, C.; Babu, M.; Nunnari, J. ER exit sites are physical and functional core autophagosome biogenesis components. Mol. Biol. Cell 2013, 24, 2918–2931.
- Tan, D.; Cai, Y.; Wang, J.; Zhang, J.; Menon, S.; Chou, H.-T.; Ferro-Novick, S.; Reinisch, K.M.; Walz, T. The EM structure of the TRAPPIII complex leads to the identification of a requirement for COPII vesicles on the macroautophagy pathway. Proc. Natl. Acad. Sci. USA 2013, 110, 19432–19437.
- Scrima, S.; Tiberti, M.; Campo, A.; Corcelle-Termeau, E.; Judith, D.; Foged, M.M.; Clemmensen, K.K.B.; Tooze, S.A.; Jäättelä, M.; Maeda, K.; et al. Unraveling membrane properties at the organelle-level with LipidDyn. Comput. Struct. Biotechnol. J. 2022, 20, 3604–3614.
- Imai, K.; Hao, F.; Fujita, N.; Tsuji, Y.; Oe, Y.; Araki, Y.; Hamasaki, M.; Noda, T.; Yoshimori, T. Atg9A trafficking through the recycling endosomes is required for autophagosome formation. J. Cell Sci. 2016, 129, 3781–3791.
- Tremel, S.; Ohashi, Y.; Morado, D.R.; Bertram, J.; Perisic, O.; Brandt, L.T.L.; von Wrisberg, M.-K.; Chen, Z.A.; Maslen, S.L.; Kovtun, O.; et al. Structural basis for VPS34 kinase activation by Rab1 and Rab5 on membranes. Nat. Commun. 2021, 12, 1564.
- Wang, J.; Menon, S.; Yamasaki, A.; Chou, H.-T.; Walz, T.; Jiang, Y.; Ferro-Novick, S. Ypt1 recruits the Atg1 kinase to the preautophagosomal structure. Proc. Natl. Acad. Sci. USA 2013, 110, 9800–9805.
- Hailey, D.W.; Rambold, A.S.; Satpute-Krishnan, P.; Mitra, K.; Sougrat, R.; Kim, P.K.; Lippincott-Schwartz, J. Mitochondria Supply Membranes for Autophagosome Biogenesis during Starvation. Cell 2010, 141, 656–667.
- Pickles, S.; Vigié, P.; Youle, R.J. Mitophagy and Quality Control Mechanisms in Mitochondrial Maintenance. Curr. Biol. 2018, 28, R170–R185.
- Vance, J.E. Thematic Review Series: Glycerolipids. Phosphatidylserine and phosphatidylethanolamine in mammalian cells: Two metabolically related aminophospholipids. J. Lipid Res. 2008, 49, 1377–1387.
- Hamasaki, M.; Furuta, N.; Matsuda, A.; Nezu, A.; Yamamoto, A.; Fujita, N.; Oomori, H.; Noda, T.; Haraguchi, T.; Hiraoka, Y.; et al. Autophagosomes form at ER–mitochondria contact sites. Nature 2013, 495, 389–393.
- De Brito, O.M.; Scorrano, L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 2008, 456, 605–610.
- Karanasios, E.; Walker, S.A.; Okkenhaug, H.; Manifava, M.; Hummel, E.; Zimmermann, H.; Ahmed, Q.; Domart, M.-C.; Collinson, L.; Ktistakis, N.T. Autophagy initiation by ULK complex assembly on ER tubulovesicular regions marked by ATG9 vesicles. Nat. Commun. 2016, 7, 12420.
