Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Shihui Liu.
NK-4 plays a key role in the treatment of various diseases, such as in hay fever to expect anti-allergic effects, in bacterial infections and gum abscesses to expect anti-inflammatory effects, in scratches, cuts, and mouth sores from bites inside the mouth for enhanced wound healing, in herpes simplex virus (HSV)-1 infections for antiviral effects, and in peripheral nerve disease that causes tingling pain and numbness in hands and feet, while NK-4 is used also to expect antioxidative and neuroprotective effects.
- NK-4
- anti-allergic
- anti-cancer
- anti-inflammatory
1. Introduction
NK-4 (1-ethyl-4-[(1Z,3E,5E)-1-(1-ethylquinolin-1-ium-4-yl)-5-(1-ethylquinolin-4-ylidene)penta-1,3-dien-3-yl]quinolin-1-ium;iodide, IUPAC name) (Figure 1) is a divalent, cationic pentamethine trinuclear cyanine dye that consists of three quinolinium rings, short N-alkyl side chains (C2), and two iodine anions [1]. It has been studied in Japan for over 100 years and has been popularly used as an over-the-counter drug since 1951. NK-4 exhibits a variety of biological activities, such as anti-allergy, anti-cancer (inhibition of cancer cell proliferation), anti-inflammation, antiviral infection, anti-oxidative, and neuroprotective effects [2]. Additionally, it has a potential to treat dilated cardiomyopathy and muscular dystrophy [2].
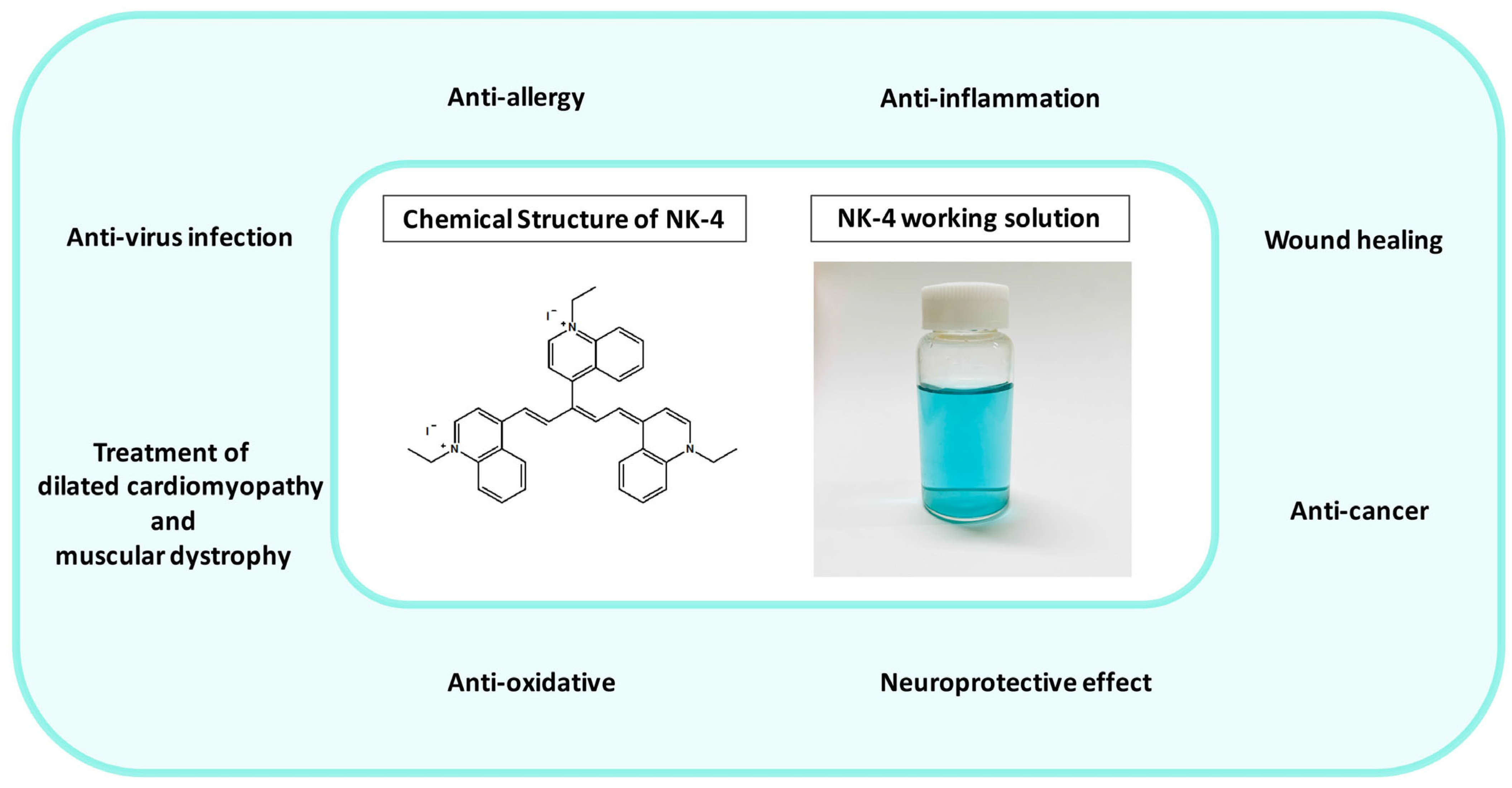
Figure 1.
Fields of application of NK-4 (in the green circle), chemical structure of NK-4, and NK-4 working solution (0.01 mg/mL).
2. Anti-Allergy
2.1. Pathogenesis and Pharmacological Therapy
Gell and Coombs’s classification divides allergies into four pathophysiological types: type I: anaphylaxis; type II: antibody-mediated cytotoxic reactions; type III: immune complex-mediated reactions; and type IV: delayed type hypersensitivity. A type I hypersensitivity is a hypersensitivity reaction that occurs within minutes after the sensitized body is exposed to the same antigen again. A type II hypersensitivity reaction is a pathological immune reaction in which IgM or IgG antibodies are combined to the corresponding antigen on the surface of target cells together with the participation of phagocytes, complement, and NK cells, leading to cell lysis or tissue damage. A type III hypersensitivity is an inflammation and tissue damage caused by the deposition of soluble immune complexes in the tissues, such as kidney, blood vessel wall, and skin, by activating the complement system; furthermore, it includes the participation of effector cells such as neutrophils and platelets, leading to cellular infiltration and localized necrosis. A type IV hypersensitivity reaction is an inflammatory reaction in which T cells are bound to corresponding antigens, leading to mononuclear cell infiltration and tissue cell damage [3].
A representative disease of the type I hypersensitivity reaction is allergic rhinitis (hay fever). Treatments of allergic rhinitis are, for instance, intranasal corticosteroids, oral and intranasal antihistamines, decongestants, intranasal anticholinergics, intranasal cromolyn, leukotriene receptor antagonists, combination therapy, immunotherapy, etc. [4].
Representative diseases of the type II hypersensitivity are Hashimoto’s disease and autoimmune hemolytic anemia (AIHA). Treatment for Hashimoto’s disease involves observation and medication, and the main therapy for Hashimoto’s disease is to control hypothyroidism, including the oral synthetic hormone levothyroxine 4 (L-T4) [5]. Prednisolone is recommended as the initial first-line treatment for primary warm AIHA. In the treatment of pathogenic B cell clones, rituximab monotherapy has become the most commonly used first-line therapy for cold AIHA. Nonpharmacological management includes thermal protection to limit hemolysis and relieve any ischemic symptoms [6].
The representative disease of the type III hypersensitivity is systemic lupus erythematosus (SLE). Treatments for SLE include use of immunomodulators (i.e., vitamin D and hydroxychloroquine), targeted therapy, and immunosuppressants [7].
The representative disease of the type IV hypersensitivity is allergic contact dermatitis. The primary treatment for allergic contact dermatitis is allergen avoidance. Databases such as the Exposure Allergen Management Program help patients choose allergen-free products. Treatment of acute exacerbations uses topical corticosteroids which are, however, not recommended to be used as a long-term treatment [8].
2.2. The Effects of NK-4
The studies investigated the immunopharmacological effects of NK-4 on type I and type IV hypersensitivity. The results demonstrated that NK-4 has a mild inhibitory effect on IgE antibody production, which is induced by heterologous passive cutaneous anaphylaxis (PCA) for 3 hours; NK-4 was shown to have a mild inhibitory effect on the homologous PCA response for 48 hours and was also demonstrated to have an inhibitory effect on the histamine release reaction by the in vitro antigen–antibody reaction in male Wistar rats (Charles River Laboratories Japan, Inc., Kanagawa, Japan). On the other hand, NK-4 significantly inhibited the cyclophosphamide (CY)-induced response from a type IV hypersensitivity reaction (delayed-type hypersensitivity (DTH)) model [1].
New Zealand white (NZB/W) F1 mice have been used as a model for autoimmune disease, such as SLE. NZB/W F1 mice produce autoantibodies such as natural thymocytotoxic autoantibodies (NTA) and anti-single DNA antibodies, and then develop into immune complex nephritis. The experiments show that NK-4 can significantly inhibit the level of NTA in the blood of NZB/W F1 mice while promoting the induction of suppressor T cells. On the other hand, NK-4 restored the anti-sheep red blood cells (SRBC) antibody response and anti-TNP-LPS PFC response in NZB/W Fl mice [9]. NK-4 exerts immunomodulatory effects by preventing T-cell damage and by directly activating dysfunctional B cells.
Moreover, previous studies have investigated whether NK-4 plays a regulatory role in Th2 cell activation and effector function. The results showed that NK-4 appears to selectively eliminate IL-4 and IL-5 production by Th2 cells that have been activated by antigen or anti-CD3ε monoclonal antibody. These phenomena have been accomplished by means of inhibiting the mRNA expression of the Th2-related transcription factors GATA-3 and NFATc1. On the regulation of Th2 cell effector function, NK-4 inhibits the secretion of eotaxin and TARC from IL-4/TNF-α-activated fibroblasts by inhibiting the STAT6 signaling pathway [10]. These results provide evidence for NK-4 as a therapeutic agent for Th2-mediated allergic inflammation.
3. Neuroprotective Effects
3.1. Pathogenesis and Pharmacological Therapy
Neurodegenerative diseases are caused by the loss of neurons, myelin sheaths, and synapses. Neurodegenerative diseases can be caused by aging and genetic mutations, and the condition of the diseases worsens over time, leading to functional impairment [79][11]. Common pathogenic mechanisms of neurodegenerative diseases include: (1) abnormal protein dynamics (protein misfolding and aggregation); (2) oxidative stress (formation of reactive oxygen species and free radicals); (3) dysfunction of neurotrophic factors; (4) mitochondrial dysfunction; (5) neuroimmune inflammation; (6) neuronal Golgi breakdown; (7) disruption of cell/axon transport; and (8) altered cell signaling. Altogether, the diversity of multiple pathogenic factors leads to multifaceted neuronal death [80][12]. The main research areas of neurodegenerative diseases include: (1) tau protein disease—Alzheimer’s disease (AD); (2) extrapyramidal disorder: Parkinson’s disease (PD), Huntington’s disease (HD); (3) spinocerebellar degeneration: multiple system atrophy (MSA); (4) autonomic disorders: Shy-Drager syndrome (SDS); and (5) motor neuron disorders: amyotrophic lateral sclerosis (ALS), Werdnig–Hoffmann disease. Ophthalmological neurodegenerative diseases mainly include retinitis pigmentosa (RP). The main drugs for neurodegenerative diseases include: galantamine, rivastigmine, and donepezil for Alzheimer’s disease [81,82,83][13][14][15]; levodopa, monoamine oxidase-B inhibitors, and dopamine agonists for Parkinson’s disease [84][16]; tetrabenazine (Xenazine) and deutetrabenazine (Austedo) for Huntington’s disease [85][17]; fingolimod (Gilenya), dimethyl fumarate (Tecfidera), and teriflunomide (Aubagio) for multiple sclerosis (MS) [86][18]; and Radicava, rilutek, exservan, nuedexta, and tiglutik for amyotrophic lateral sclerosis [87][19]. As a therapeutic drug for retinitis pigmentosa, Luxturna® (voretigene neparvovec) is the only Food and Drug Administration (FDA)-approved retinitis pigmentosa therapy, designated for a small subset of patients with RPE65 mutations [88][20]. On 23 June 2022, the FDA published a 5-year action plan for drugs of neurodegenerative diseases, focusing on ALS [89,90][21][22]. Therefore, with the deepening of neurodegenerative disease research, multi-pathway and multi-target therapeutic drugs urgently need to be developed.3.2. The Effects of NK-4
In a report, besides the neurotrophic and neurogenesis activity of NK-4 observed in a transgenic mouse model of Alzheimer’s disease (Tg 2576), the effect of NK-4, which was better than acetylcholinesterase inhibitors (AChEIs), was also observed in the early stages of mouse dementia (6 months old). NK-4 may be a new drug for the treatment of early- to late-stage Alzheimer’s disease [91][23]. Another study showed that NK-4 had neurotrophin-like activity and exhibited neuroprotective effects in vitro and in vivo. In vitro, NK-4 significantly enhanced nerve growth factor (NGF)-induced neurite outgrowth in PC12HS cells. In vivo, NK-4 effectively prevented injury in a rat stroke model (middle cerebral artery occlusion (MCAO) Rats) through neurotrophin-like activity and antioxidative activity [75][24]. In vitro, NK-4 was shown to dose-dependently protect PC12 cells from oxidative stress-induced toxicity by 6-hydroxydopamine (6-OHDA) or hydrogen peroxide (H2O2). In an ataxia animal model (Syrian hamster marked by Purkinje cell degeneration, PCD model) of neurodegeneration, the studies showed that the neuroprotective effects of NK-4 are mediated by the PI3K-Akt signaling pathway [92][25]. NK-4 can also reduce the accumulation of Aβ in the brain, inhibit Aβ aggregation, scavenge free radicals, and produce neuroprotective effects by its intraperitoneal injection in Alzheimer’s disease model AβPP transgenic mice (Tg2576). It is thus suggested that NK-4 can also be used to treat Alzheimer’s disease [77,93][26][27]. In a recent study, researchers administered NK-4 into the eyes of RCS rats via intravitreal injection; the researchers found that NK-4 could inhibit the apoptosis of photoreceptor cells. Hmox1, Mt1, Atf5, Slc7a11, and Bdh2 genes were up-regulated by the RNA-seq analysis and confirmed by the RT-PCR analysis. Functional and pathway enrichment analyses of up-regulated genes in that study suggest that the neuroprotective effect of NK-4 in RCS rat retina might be related to the retinal pigment epithelial metabolic process, transition metal ion homeostasis, and negative regulation of neurons’ apoptosis by Metascape analysis. They also uploaded five genes (Hmox1, Mt1, Slc7a11, Bdh2, and Atf5) to the DAVID database for the functional annotation clustering of bioinformatics resources. Based on the gene function distributed by DAVID, it was divided into the following categories: response to oxidative stress, negative regulation of neuron apoptotic process, and iron ion homeostasis [78][28]. All of these results revealed the molecular mechanism by which NK-4 inhibits the apoptosis of photoreceptor cells, indicating that NK-4 upregulates genes involved in anti-oxidative stress and anti-apoptotic pathways.9. Synthesis of NK-4
The synthetic route for NK-4 is shown in Figure 42 [94,95][29][30]. 1-Ethyl-4-methylquinolin-1-ium iodide was treated with Vilsmeier reagent generated in situ from P(O)Cl3 and N,N-dimethylformamide, affording the desired NK-4 at a 41% yield (Figure 42A). A different C1 unit protocol was also reported (Figure 42B). However, these routes could be used for the same three-quinoline moiety, but not for hetero-quinoline moieties. In the future, the development of a new synthetic methodology is highly required to supply various derivatives which have different quinoline cores (Figure 42C).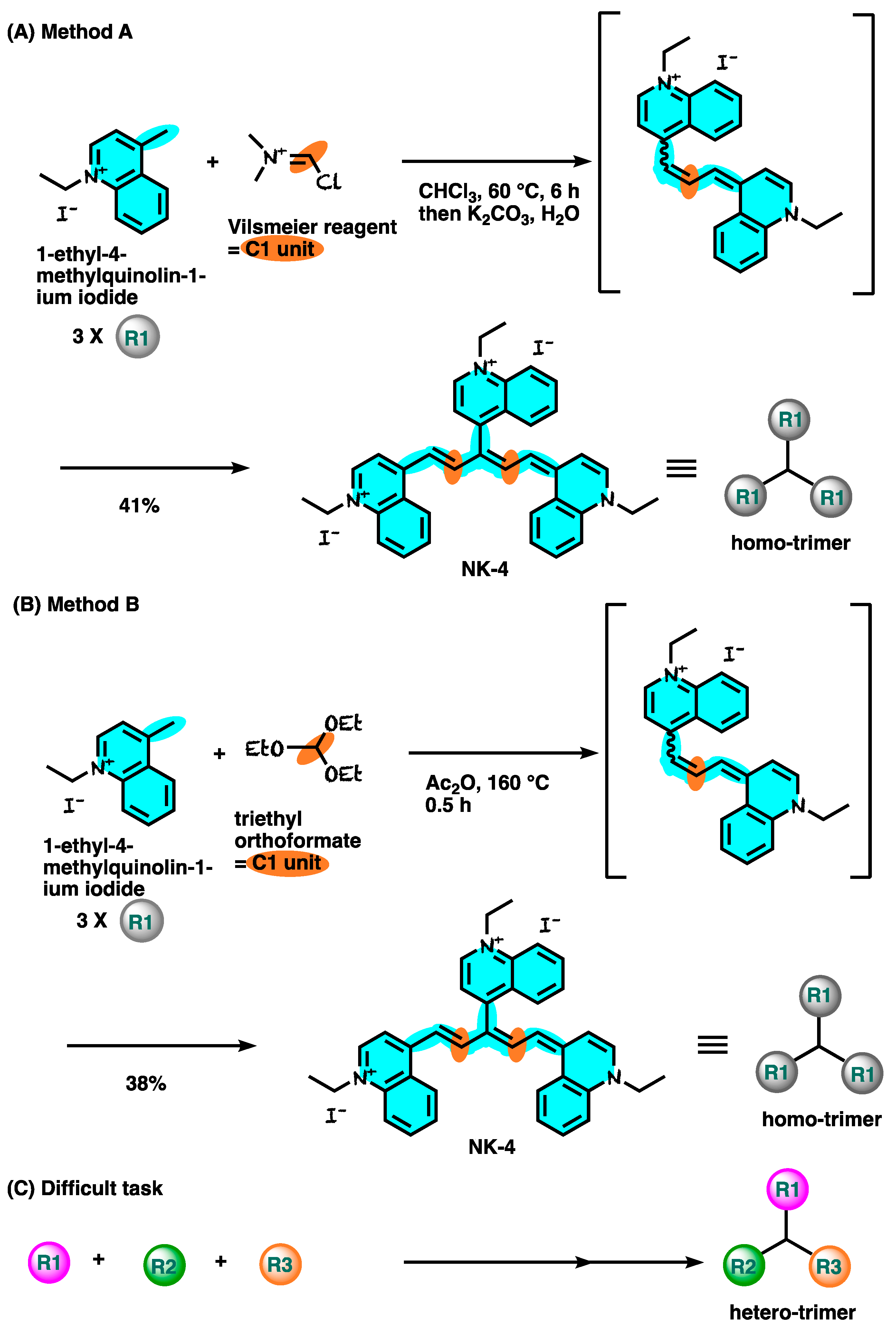
Figure 42.
Synthetic route for NK-4.
References
- Sasaki, Y.; Nagai, N.; Okimura, T.; Yamamoto, I. Immunopharmacological actions of lumin (I): Anti-allergic actions of lumin. Nihon Yakurigaku Zasshi 1987, 89, 1–7. (In Japanese)
- Iwata, Y.; Katayama, Y.; Okuno, Y.; Wakabayashi, S. Novel inhibitor candidates of TRPV2 prevent damage of dystrophic myocytes and ameliorate against dilated cardiomyopathy in a hamster model. Oncotarget 2018, 9, 14042–14057.
- Descotes, J.; Choquet-Kastylevsky, G. Gell and Coombs’s classification: Is it still valid? Toxicology 2001, 15, 43–49.
- Sur, D.K.; Plesa, M.L. Treatment of Allergic Rhinitis. Am. Fam. Physician 2015, 92, 985–992.
- Ralli, M.; Angeletti, D.; Fiore, M.; D’Aguanno, V.; Lambiase, A.; Artico, M.; de Vincentiis, M.; Greco, A. Hashimoto’s thyroiditis: An update on pathogenic mechanisms, diagnostic protocols, therapeutic strategies, and potential malignant transformation. Autoimmun. Rev. 2020, 19, 102649.
- Berentsen, S.; Barcellini, W. Autoimmune Hemolytic Anemias. N. Engl. J. Med. 2021, 385, 1407–1419.
- Fava, A.; Petri, M. Systemic lupus erythematosus: Diagnosis and clinical management. J. Autoimmun. 2019, 96, 1–13.
- Nassau, S.; Fonacier, L. Allergic Contact Dermatitis. Med. Clin. N. Am. 2020, 104, 61–76.
- Sasaki, Y.; Nagai, N.; Okimura, T. Immunopharmacological actions of lumin (II): Effect of lumin administration in NZB X NZW (B/W) F1 mice. Nihon Yakurigaku Zasshi 1987, 89, 9–13. (In Japanese)
- Kohno, K.; Koya-Miyata, S.; Harashima, A.; Ariyasu, T.; Ushio, S. NK-4 exerts selective regulatory effects on the activation and function of allergy-related Th2 cells. PLoS ONE 2018, 13, e0199666.
- Butler, C.A.; Popescu, A.S.; Kitchener, E.J.A.; Allendorf, D.H.; Puigdellívol, M.; Brown, G.C. Microglial phagocytosis of neurons in neurodegeneration, and its regulation. J. Neurochem. 2021, 158, 621–639.
- Jellinger, K.A. Basic mechanisms of neurodegeneration: A critical update. J. Cell. Mol. Med. 2010, 14, 457–487.
- Heinrich, M. Chapter 4—Galanthamine from galanthus and other amaryllidaceae—Chemistry and biology based on traditional use. In The Alkaloids: Chemistry and Biology; Cordell, G.A., Ed.; Academic Press: Waltham, MA, USA, 2010; Volume 68, pp. 157–165.
- Durães, F.; Pinto, M.; Sousa, E. Old drugs as new treatments for neurodegenerative diseases. Pharmaceuticals 2018, 11, 44.
- Li, D.D.; Zhang, Y.H.; Zhang, W.; Zhao, P. Meta-analysis of randomized controlled trials on the efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer’s disease. Front. Neurosci. 2019, 13, 472.
- Löhle, M.; Reichmann, H. Controversies in neurology: Why monoamine oxidase B inhibitors could be a good choice for the initial treatment of Parkinson’s disease. BMC Neurol. 2011, 11, 112.
- Claassen, D.O.; Carroll, B.; De Boer, L.M.; Wu, E.; Ayyagari, R.; Gandhi, S.; Stamler, D. Indirect tolerability comparison of Deutetrabenazine and Tetrabenazine for Huntington disease. J. Clin. Mov. Disord. 2017, 4, 3.
- Guarnera, C.; Bramanti, P.; Mazzon, E. Comparison of efficacy and safety of oral agents for the treatment of relapsing-remitting multiple sclerosis. Drug Des. Dev. Ther. 2017, 11, 2193–2207.
- Available online: https://www.als.org/navigating-als/living-with-als/fda-approved-drugs (accessed on 1 September 2022).
- Cross, N.; van Steen, C.; Zegaoui, Y.; Satherley, A.; Angelillo, L. Current and Future Treatment of Retinitis Pigmentosa. Clin. Ophthalmol. 2022, 16, 2909–2921.
- Available online: https://www.fiercebiotech.com/biotech/fda-unveils-5-year-action-plan-neurodegenerative-drugs-focus-als (accessed on 1 August 2022).
- Available online: https://www.fda.gov/news-events/public-health-focus/accelerating-access-critical-therapies-als-act-act-als (accessed on 1 August 2022).
- Ohta, H.; Akita, K.; Arai, S.; Ohta, T.; Kawata, T.; Fukuda, S. P3-396: NK-4, a photosensitizing cyanine dye, prevented beta-amyloid-induced cognitive impairment in a transgenic mouse model of Alzheimer’s disease. Alzheimer’s Dement. 2010, 6, S568.
- Koya-Miyata, S.; Ohta, H.; Akita, K.; Arai, S.; Ohta, T.; Kawata, T.; Fukuda, S. Cyanine dyes attenuate cerebral ischemia and reperfusion injury in rats. Biol. Pharm. Bull. 2010, 33, 1872–1877.
- Ohta, H.; Arai, S.; Akita, K.; Ohta, T.; Fukuda, S. Neurotrophic effects of a cyanine dye via the PI3K-Akt pathway: Attenuation of motor discoordination and neurodegeneration in an ataxic animal model. PLoS ONE 2011, 6, e17137.
- Ohta, H.; Akita, K.; Ohta, T. Effects of NK-4, a cyanine dye with antioxidant activities: Attenuation of neuronal deficits in animal models of oxidative stress-mediated brain ischemia and neurodegenerative diseases. Biol. Pharm. Bull. 2012, 35, 1831–1835.
- Ohta, H.; Arai, S.; Akita, K.; Ohta, T.; Fukuda, S. Effects of NK-4 in a transgenic mouse model of Alzheimer’s disease. PLoS ONE 2012, 7, e30007.
- Liu, S.; Matsuo, T.; Miyaji, M.; Hosoya, O. The effect of cyanine dye NK-4 on photoreceptor degeneration in a rat model of early-stage retinitis pigmentosa. Pharmaceuticals 2021, 14, 694.
- Jones, G.; Stanforth, S.P. The Vilsmeier reaction of non-aromatic compounds. Org. React. 2000, 56, 356645.
- Mizuno, Y.; Tanabe, Y. Syntheses of cyanine dyes. IX. Further notes on chemical structure of neocyanine. Yakugaku Zasshi 1953, 73, 227–231. (In Japanese)
More
