Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Arnav Goel and Version 3 by Conner Chen.
Biopolymers are an emerging class of novel materials with diverse applications and properties such as superior sustainability and tunability. Biopolymers pose another option for novel energy material applications. Biopolymers are biodegradable macromolecules composed of repeating units. Natural biopolymers are derived from living matter, such as proteins, polysaccharides, and nucleic acids.
- protein
- polysaccharide
- biopolymer
- battery
1. Introduction
Batteries and electric storage devices have become ubiquitous in human lives and can be found in cars, phones, and most portable devices. Population growth and increasing demand have propelled advancements to produce cleaner, smaller, and cheaper renewable energy sources [1]. The growing prevalence of implantable energy storage devices in biotechnology calls for increased stability and energy density to maximize the lifespan and minimize the size of the device [2]. Considering the array of uses in the human body (e.g., cochlear implants, implantable defibrillators, and drug delivery systems), an equally diverse array of batteries have been engineered to power these devices [2][3][2,3].
Lithium-ion batteries have been utilized extensively due to their high charge density, high coulombic efficiencies, and low self-discharge properties [4]. They are widespread amongst rechargeable portable devices, such as cellphones and computers, as well as electric vehicles [5]. Lithium-ion batteries are composed of a cathode, an anode, a separator, and an electrolyte [6]. The cathodes and anodes carry charge, the separator insulates both the electrodes from each other allowing Li+ ions to flow to each electrode, and the electrolyte carries ions [6][7][6,7]. Electrode compositions have been optimized to maximize energy density, as many electrodes contain lithium metal oxides with amounts of nickel, cobalt, and manganese, iron, or titanium [5][7][5,7]. Unfortunately, these electrodes suffer from poor electrical conductivity despite high energy density [7]. Furthermore, lithium-ion batteries are susceptible to hazardous failure due to electrochemical instability.
A new frontier in 2-dimensional allotropic materials allows for the development of novel electrode solutions. Two-dimensional materials such as graphene, transition metal dichalcogenides such as molybdenum disulfide (MoS2), and transition metal carbides such as titanium carbide (Ti3C2), enable rapid diffusion of ions, enhancing their effectiveness as electrodes for metal ion batteries [8]. For example, borophene is similar to graphene, but is created using the physical vapor deposition (PVD) technique, utilized by vaporizing boron so that it deposits onto a thin sheet of silver [9][10][9,10]. Despite naturally occurring as a metalloid, when boron is in a two-dimensional form as in borophene, it behaves as a highly conductive metal [10]. Borophene has been demonstrated to be an effective anode for lithium batteries without energy dispersion during the charge/discharge process, ensuring a quick charging time and long lifespan [10][11][10,11].
Biopolymers pose another option for novel energy material applications. Biopolymers are biodegradable macromolecules composed of repeating units. Natural biopolymers are derived from living matter, such as proteins, polysaccharides, and nucleic acids. Frequently utilized natural biopolymers include silk, hyaluronic acid, collagen, pectin, and gelatin [12][13][14][12,13,14]. Interestingly, their increase in demand stems from both a cost and toxicity standpoint as an alternative to petroleum-based plastics [15][16][17][15,16,17]. Specifically, the manufacturing, usage, and disposal of common plastics such as polystyrene (PS), polypropylene (PP), and polyethylene terephthalate (PETE) contribute to landfill consumptions, higher greenhouse gas emissions, and pollution among other negative effects [18][19][18,19]. Despite the significant established infrastructure and thus convenience of fossil-based plastics, biopolymers present a unique solution to enabling sustainability and biodegradability in materials [20][21][20,21]. They are further highlighted by their biocompatibility, low production cost, availability, and high control in fabrication [22]. Electronics typically struggle at their end-of-life due to improper disposal resulting in a health and environmental hazard from toxic leakages [23]. This lack of safe degradation originates from relatively stable bonds present in the material. For batteries, this includes potentially toxic elements such as lead, lithium, cadmium, among other metals [24]. Meanwhile, biopolymer-based electrical devices, previously synthesized from materials such as cellulose acetate, silk, chitosan, and pectin all exhibit the unique advantage of biodegradability from both mechanical breakdown and enzymatic cleavage of susceptible bonds via microorganisms [25]. These same functional groups can also promote the customization of biopolymers. For example, Lim et al. utilizes porous carbons with chitosan for lithium-ion batteries to increase efficiency of battery cycles [26]. In addition, biopolymers can demonstrate variable mechanical properties via control of elastic modulus, allowing higher densities of energy storage in previously incapable chemistries [27]. Many biopolymers further exhibit valuable electrical properties such as conductivity and ion conductance [28][29][30][28,29,30].
Based on the aforementioned advantages of biopolymers, many prior studies have focused on energy applications for supercapacitors, rechargeable batteries, solar cells, and fuel cells [31]. Supercapacitors are a promising alternative to traditional batteries; compared with normal capacitors, their higher capacitance allows them to be a potential large storage form for energy. Biopolymer research in this regard is focused on electrode materials with good conductivity, large surface area, and many active sites [32]. Several studies have focused on composite materials integrating graphene with biopolymers [32]. Combinations of this type include binary composites (e.g., graphene with polyindole [33]), tertiary composites (e.g., graphene, silicon dioxide, and polyaniline [34]), and quaternary composites (e.g., graphene, platinum, carbon nanotubes, and polyaniline [35]). Additionally, biopolymers are a promising possibility for innovation in rechargeable batteries. Polyethylene oxide-based lithium metal polymer batteries have improved flexibility and processability but currently, their lower ionic conductivity limits them in comparison with state-of-the-art lithium-ion batteries [36]. Other potential avenues for biopolymer-based rechargeable batteries include composite polymer electrolytes and hybrid inorganic-organic electrolytes [36]. Furthermore, biopolymers have also been found to be an exciting source for improvement in solar cells, specifically in the application of dye-sensitized solar cells. Dye-sensitized solar cells (DSSCs), relying on organic dyes for the conversion of light energy to electrical energy, are a cheaper, lighter alternative to conventional solar cells [37]. Polysaccharide biopolymers have been investigated as electrolytes for DSSCs, with examples including cellulose, agarose, chitosan, starch, carrageenan, with gelatin a non-polysaccharide electrolyte option [38]. Biopolymers have been explored in fuel cell applications as proton and ion exchange membranes. Nafion is a prominent proton exchange membrane currently; however, it faces limitations due to high costs and methanol crossover [39]. Cellulose, chitin, and alginate-based membranes offer lower monetary and environmental costs in comparison with nafion in proton exchange membranes with cellulose and chitin-based membranes showing more promise for anion exchange membrane options [40].
2. Biopolymer Materials
2.1. Silk
Silk is one of the oldest protein-based biopolymers harnessed by mankind, utilized for a variety of functions especially in the textile industry (Table 1) [41][59]. Silk is produced by silkworm (Bombyx mori), orb-weaver spiders, and numerous organisms of the order Lepidoptera as seen in Figure 12 [42][60]. The structure of silk directly correlates to its unique properties such as biocompatibility, biodegradability, mechanical strength, and precise control in fabrication [43][61]. Silk is a highly crystalline material, composed of repeating amino acid motifs in its primary structure and compact β sheet regions in its secondary structure [44][62]. Silk is further broken down into its unique variants, most notably silk I (with helices/coils) and silk II (with β sheets). The distinct crystal formations between silk I (monoclinic unit cell) and silk II (orthorhombic) result in a dimorphic crystalline structure that is a prerequisite for piezoelectric materials [45][63].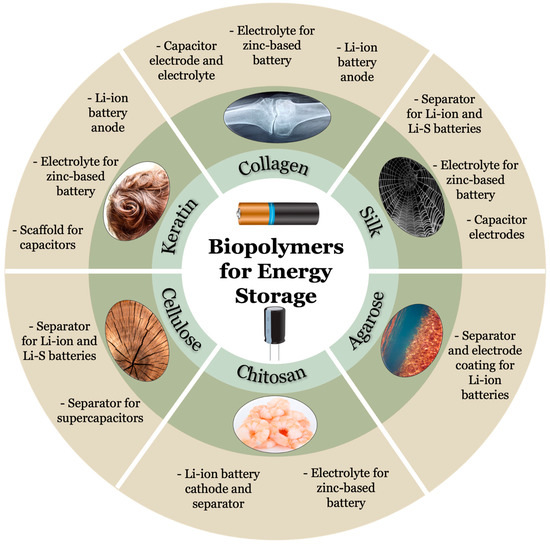
Figure 12. Source and applications for biopolymers commonly utilized for energy storage purposes such as batteries and capacitors. Keratin, collagen, and silk are protein-based biopolymers while cellulose, chitosan, and agarose are polysaccharide-based biopolymers. (Images of biopolymers were obtained with permission from pixabay.com, accessed on 1 December 2022).
2.2. Keratin
Keratin is a protein-based biopolymer known for its robust mechanical properties and self-assembling capability [46][64]. Keratins are classified as “hard”, stemming from the hair and “soft”, deriving from epithelial tissue. Hard keratins are typically associated with higher contents of the cysteine residue, yielding higher degrees of sulfur-based crosslinking [47][65]. Thus, as a fibrous protein, keratins are very stable polymers and insoluble. Furthermore, keratins are divided into α-keratins and β-keratins. α-keratins are found to be a tightly coiled right-handed helix with 3.6 amino acids per turn. This tertiary structure is established via hydrogen bonding in the side chains. Generally, α-keratin is the primary material in hair and wool among others. Meanwhile, β-keratins are found as pleated sheets, stabilized via hydrogen bonding between the carboxyl and amino groups in respective residues. β-keratin is usually located in reptiles and birds and is notoriously challenging to extract [48][66]. For energy applications, keratins are incredibly useful due to the presence of nitrogen atoms that enhance carbon activation for ion transport as well as inherent biopolymer properties such as biodegradability [49][67].2.3. Collagen
Collagen is a protein-based polymer serving as the primary structural protein component in biological systems [50][68]. Collagen is known for its fibrous, hierarchical composition that ultimately yields a triple coil helix for the quaternary structure known as tropocollagen. These tropocollagens are then bundled to form collagen fibers. This novel polymer design provides collagen excellent mechanical properties including elasticity and strength [51][69]. Similar to previous polymers, collagen is rich in various atoms such as nitrogen, oxygen, and especially carbon. This translates into applications for doping in order to further induce electrochemical properties into biopolymers [52][70]. Interestingly, collagen for electrical applications is often sourced from leather waste, highlighting the importance of reusability in this search for more sustainable energy solutions [53][71].2.4. Chitosan
Chitosan is a derivative of chitin, sourced from the exoskeleton of many insects, arthropods, shellfish, and even cell walls of fungi as portrayed in Figure 12 [54][72]. As a polysaccharide-based biopolymer, chitin’s structure is composed of β-1,4-glycosidic linked 2-acetamido-d-glucose and 2-amino-d-glucose units. Chitosan is formed by eliminating acetate through the chemical reaction of the previous polymer [55][73]. Notably, chitosan is seen as an evolutionary intermediate between cellulose and collagen [56][74]. However, unlike the latter examples, chitosan has a significantly higher percentage of nitrogen atoms, adding functionality as a chelating agent for electrical applications especially as additives within electrodes [57][75].2.5. Cellulose
Cellulose, the most abundant biomaterial, is found mainly in plants, algae, and some species of bacteria [58][76]. Cellulose is a polymer fabricated of 1,4 covalent glycosidic bonded β-D glucose monomers and is insoluble despite its hydrophilic nature [59][77]. When synthesized in nature, cellulose is typically found as a structure of elementary fibrils which form microfibrils which then form macro cellulose fibers [60][78]. The cellulose fibers typically arrange as a semicrystalline structure in which regions of ordered chains are interrupted by amorphous regions. Four crystalline allomorphs of cellulose exist, cellulose I, II, III, IV. Cellulose I is fabrictaed of a mix of triclinic (Iα) and monoclinic (Iβ) structures and is obtained naturally [61][79]. Cellulose II, the most stable form of cellulose, can be obtained from cellulose I by regeneration or mercerization of cellulose I [62][80]. Cellulose III type I can be obtained from treatment of cellulose I and can be used to form cellulose IV type I; cellulose III type II is produced from cellulose II and can be utilized to form cellulose IV type II [62][80]. Cellulose can also be modified chemically to form cellulose derivatives with slightly distinct characteristics [63][64][65][66][81,82,83,84]. Cellulose acetate, produced by replacement of hydroxyl groups in glucose with acetyl groups, can be obtained from agricultural byproducts and does not require further chemical or mechanical treatment [62][80]. Carboxymethyl cellulose, derived from cellulose by hydroxyl group substitution for carboxymethyl group, is more soluble in water and other organic solvents and is effective for the formation of foams and aerogels [67][85]. This ease in modification in addition to chemical stability render cellulose a potent option for separators in energy storage options [68][86].2.6. Agarose
Agarose, a linear biomass-derived polysaccharide, is composed of the repeating monomer units of agarobiose fabrictaed of β-D-galactose and α-3,6-lactose-L-galactose [69][87]. Agarose is typically obtained by extraction from seaweed as suggested in Figure 12 [70][88]. Characteristics of note are its hydrophilicity, chemical stability, and electrical neutrality [71][89]. Agarose may be insoluble in room temperature water, but can dissolve in ninety degree Celsius water, allowing for manipulation without harsh reagents [72][90]. The ether and hydroxyl functional groups aid in directing ion transfer. Agarose is gelated by self-assembly mainly due to hydrogen bond formation, resulting in a porous structure [73][91]. Such porosity enables agarose to be an ideal candidate for electrode materials.Table 1.
Overview of natural biopolymers as energy storage materials.
| Biopolymer | Natural Source | Characteristics | Applications |
|---|---|---|---|
| Silk | Silkworms, spiders [42][60] | Highly crystalline, biocompatible, biodegradable, high mechanical strength [43][61]. | Separator for lithium ion [74][92] and lithium sulfur batteries [75][93]. |
| Keratin | Hair, wool (α), reptiles (β) [48][66] | Stable, self-assembling, strong mechanical properties [47][65]. | Anode in lithium ion batteries [76][94], electrolyte in zinc-based batteries [77][95], scaffold in supercapacitors [78][96]. |
| Collagen | Leather waste [53][71] | Fibrous, excellent elasticity and strength [51][69]. | Anode in lithium ion batteries [79][97], electrolyte in zinc-based batteries [80][98], separator in supercapacitors [81][99], electrode in supercapacitors [82][100]. |
| Chitosan | Exoskeletons, fungi cell walls [54][72] | Biocompatible, biodegradable, functional as a chelating agent [57][75]. | Additive for cathode and separator in lithium sulfur batteries [83][101], electrolyte in zinc-based batteries [84][102]. |
| Cellulose | Plants, tunica, algae, bacteria [62][80] | High mechanical strength, high degrees of polymerization and crystallinity [64][82]. | Separator in supercapacitors [85][103] and lithium-ion batteries [86][104]. |
| Agarose | Seaweed [70][88] | Chemically stable, electrically neutral [71][89]. | Separator [87][105] and anode coating material [70][88] in lithium-ion batteries |
