Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Nina Filip and Version 2 by Conner Chen.
Lung cancer remains a major public health problem both in terms of incidence and specific mortality despite developments in terms of prevention, such as smoking reduction policies and clinical management advances. Better lung cancer prognosis could be achieved by early and accurate diagnosis and improved therapeutic interventions. Nanotechnology is a dynamic and fast-developing field; various medical applications have been developed and deployed, and more exist as proofs of concepts or experimental models.
- nanomedicine
- lung cancer
- drugs
1. Introduction
The birth of the term nanotechnology is anecdotally linked to the American physicist Richard Feynman in the early 1960s; currently, this is an umbrella term for technologies dealing with structures between 1 and 100 nanometers [1]. Nanotechnology has established a foothold in the medical space; it is employed in various branches spanning from diagnosis to treatment. Atheroma plaque healing, regenerating damaged nerves, and targeting tumor tissues are only a few examples of the practical applications of nanotechnology [2].
Recent years have brought significant improvements concerning survival and quality of life for various hematological and solid malignant disease patients; lung cancer lags behind despite new emerging therapies [3]. Various strategies have been devised to improve lung cancer outcomes; early diagnosis and better therapeutic options are focus points. Early diagnosis enables radical therapeutic procedures such as surgical resection or curative intent radiotherapy while maintaining quality of life; however, in the case of lung cancer, late onset of clinical signs, lack of reliable biomarkers, and imaging-related limitations make such an approach difficult [4].
Lung cancer therapeutic protocols are usually chosen considering the histology of the tumor and the extension and mainly combine surgery, radiation therapy, and chemotherapy. For non-resectable lung cancer, the prognosis is linked to oncological treatment efficacy, which is generally limited by tolerability and toxic side effects [5]; immuno- and targeted therapy are recent additions gaining ground and leading towards personalized medicine [6] but are adequate only in a minority of cases.
Nanotechnologies could alleviate some conventional therapy drawbacks and improve efficacy; tailoring drug pharmacokinetics (by facilitating intra- and intercellular traffic or navigating the tumor micro-environment), targeting various cellular lines, and modulating the immune response are only a few possibilities [7].
Nanoparticles is an umbrella term encompassing a plethora of chemically different structures potentially useful in both early diagnosis and better therapy.
2. Nanotechnologies and Lung Cancer Therapy
Lung cancer remains one of the most frequently diagnosed malignant diseases; despite some progress in prevention, early detection, and advanced therapy, its prognosis is usually severe, and associated mortality remains high. Tobacco smoking was identified as the main risk factor, and some population-level risk mitigation measures have been implemented; other external factors, such as air pollution (environmental and domestic), also have a role [5]. Surgery, radiotherapy, and oncological therapies are the main pillars of lung cancer treatment; early diagnosis and correct staging are paramount to optimize outcomes [6]. Classic oncological management of lung cancer involves chemotherapy, though recent molecular biology developments have brought out new therapeutic methods with increased efficacy and better safety profiles, such as targeted agents and immunotherapy. Paclitaxel is a chemotherapic agent frequently used in breast, ovary, prostate, and lung cancer protocols. The doublet paclitaxel platinum salt may be considered the mainstay of non-small cell lung cancer therapy. Paclitaxel acts as a tubulin-binding agent, stopping mitosis and promoting cellular death; it has low hydro-solubility, and therefore, an emulsifier vehicle is necessary, usually the solvent oil cremophor ethanol (CrEL). The administration of a CrEL-paclitaxel formula may be followed by potentially lethal adverse events such as hypersensitivity reactions, peripheral neuropathy, or myelosuppression [8][9][10][103,104,105]. Various mitigation strategies are used in clinical settings, such as corticoid and antihistamine premedication and low infusion rates; one potential alternative may be the use of albumin-bound paclitaxel nanoparticles, which seem to have increased plasma life and antitumor activity, at least in murine human tumor xenograft models [11][106]. The nab-paclitaxel formula (under the trade name Abraxane) was initially FDA-approved in 2004 for metastatic breast cancer; in 2012, it was accepted for the first-line treatment of locally advanced or metastatic non-small cell lung cancer in combination with carboplatin in patients who are not candidates for curative surgery or radiation therapy [12][107]. Using the nab-paclitaxel form allowed enhanced tumor penetration and cellular uptake [13][108] (probably by transporter-mediated mechanisms) with a higher clinical response rate and a better safety profile than classic CrEL paclitaxel [14][109]. There are data supporting the use of nab-paclitaxel as higher effective concentrations can be reached with a shorter infusion time, eliminating the need for premedication used to alleviate the risk of solvent-induced hypersensitivity reactions [15][110]. Another way to improve the chemical stability and solubility of CrEL-paclitaxel made use of liposomes; the cytotoxic effect was similar to classic Taxol, but bioavailability and stability were improved. In 2006, a formulation was approved in China under the trade name LIPUSU [16][86]. There are data suggesting that higher cellular uptake and better cytotoxicity with a similar safety profile may be attained by altering the lipid components of the liposomes (by adding lysophosphatidylcoline by a simple process) [17][111]. Other paclitaxel nanoparticles are under scrutiny. Polymeric micellar paclitaxel (pm-Pac) is a CrEL-free structure that was recently tested in phase III trials, showing increased tumor cell penetration and reduced adverse effects in combination with cisplatin, thus potentially becoming a new chemotherapy option for advanced non-small-cell lung cancer patients [18][19][112,113]. Polylactic-co-glycolic acid (PLGA) has also been considered as a potential paclitaxel carrier; there are published data supporting higher cytotoxicity, stronger apoptosis signal, weaker migration and invasion for NSCLC cells when using solvent-based paclitaxel as a comparator [20][21][114,115]. Doxorubicin is a potentially useful chemotherapeutic agent for many solid tumors [22][23][116,117], but its high toxicity and induced resistance may impose limits on its use. Particularly for lung cancers, doxorubicin shows low cellular penetration, low tumor concentrations, and significant toxicity [24][25][118,119]. Various nanoparticle–doxorubicin delivery systems have been tested, and some showed better pharmacokinetics and bioavailability and a lower effect on normal cells [20][26][95,114]. The dimercaptosuccinic acid terminated poly (amido-amine) (PAMAM) dendrimers conjugated with doxorubicin proved effective in delivering doxorubicin using a glucose moiety as a targeting structure and making use of the increased glucose uptake of tumor cells; furthermore, the dimensions of the conjugates decreased the renal elimination and demonstrated a longer half-life [27][120]. Doxorubicin-containing PEGylated liposomes are available and widely used in clinical oncology (PLD; CAELYX, Schering-Plough Corp., Kenilworth, NJ, USA/DOXIL, ALZA, Mountain View, CA, USA) as toxic effects (mainly cardiotoxicity and myelosuppression but also vomiting and alopecia) are mitigated compared with conventional doxorubicin [20][28][29][114,121,122]. Molecular cancer targets are products of so-called driver mutations. The most frequent are epidermal growth factor receptor (EGFR), KRAS (Kirsten rat sarcoma virus gene), HER2 (human epidermal growth factor receptor 2 gene), ALK (anaplastic lymphoma kinase), ROS1 (tyrosine-protein kinase ROS gene), cMET (MNNG HOS transforming gene), BRAF (B-Raf gene), RET (rearranged during transfection gene), and NTRK (neurotrophic tyrosine receptor kinase gene) [30][123]. The best-known driver mutations for NSCLC involve the epidermal growth factor receptor gene (EGFR), being detected in 10–15% of lung adenocarcinoma patients. EGFR is a receptor tyrosine kinase, a member of the ErbB family, and may be physiologically activated by multiple ligands; this interaction may activate various intracellular signaling pathways, such as PI3K/AKT/mTOR, Ras/Raf/MEK/ERK1/2, and the phospholipase C (PLC) cascade—with clear implications regarding cell migration, attachment, angiogenesis, and organogenesis regulation [30][31][123,124]. Various activating EGFR mutations have been documented; the in-frame exon 19 deletion and the L858R substitution account for 85% of relevant driver mutations in 85% of NSCLC cases, but there are multiple deletions, insertions, point mutations and duplications reported concerning exons 18–25 [32][125]. Such mutations may lead to persistent signal pathway activation with decreased apoptosis and cell proliferation and play a role in tumorigenesis; therefore, the EGFR domains became a potential target for novel antitumor agents [33][126]. The first generation of EGFR tyrosine kinase inhibitors (EGFR-TKI) has a reversible effect on the tyrosine kinase EGFR domain. Various clinical trials have shown improved survival for mutation-harboring patients using standard cytotoxic therapy as a comparator [34][35][127,128]. Although the survival rate has improved, the patients acquire resistance to these drugs after 9–14 months [36][37][129,130]. EGFR exon 20 T790M deletion, which occurs in 50–60% of NSCLC patients undergoing first-generation EGFR-TKI therapy (such as erlotinib or gefitinib), is the most common mechanism of acquired resistance; the second generation of EGFR-TKI (afatinib and dacomitinib) was developed aiming to circumvent this drawback without noticeable success [38][131]. The 3rd-generation EGFR-TKI (osimertinib, rocelitinib, olmutinib) proved to be effective in overcoming the resistance induced by the T790M deletion and are currently considered first-line agents in NSCLC protocols for patients with driver mutations [39][132]. Similar to classic antitumor agents, the idea of boosting the effects of EGFR-TKIs using nanoparticles was investigated. There are data concerning the use of GEF-loaded poly(ε-caprolactone)-poly(ethyleneglycol)-poly(ε-caprolactone) (PCEC)-bearing nanoparticles (GEF-NPs) with improved antitumor effects, prolonged survival time, and less side effects using classic gefitinib as a comparator [40][133]. Human serum albumin (HAS) is non-immunogenic and has ideal biocompatibility. It is frequently used as a drug vehicle as it improves the solubility of lipophilic drugs. Hyaluronic acid (HA) is a negatively charged polysaccharide that is similarly biocompatible and known to interact with some surface molecules such as CD-44, lymphatic vessel endothelial receptor-1, and receptor for hyaluronan-mediated motility that are frequently overexpressed in malignant cells [41][42][134,135]. An erlotinib/hyaluronic acid/human serum albumin complex (ERT-HSA-HA NPs) was developed and tested on tumor cell lines and animal models with promising results, including tumor growth inhibition and lack of recurrence, possibly explained by longer plasma half-life and higher tumor uptake [43][44][136,137]. Complex associations were also tested. Doxorubicin and icotinib (proven more effective than erlotinib and apatinib) were encapsulated using cationic amphipathic starch and hyaluronic acid. The resulting NPs were tested using lung cancer lines and murine models and were shown to accumulate in tumor cells with a smaller effect on normal cells [45][10]. Both afatinib and dacomitinib (a second-generation, irreversible EGFR-TKI, FDA-approved) have low solubility, which translates to low pulmonary tissue bioavailability; such a drawback might be circumvented by a direct administration route by using a system of poly-(lactic-co-glycolic-acid) nanoparticles (PLGA NPs) developed for inhalation for pulmonary lesions [46][138]. Osimertinib is the first FDA-approved third-generation EGFR-TKI; current therapeutic protocols allow its use for both NSCLC patients with activating EGFR mutation and patients with T790M resistance mutation cancers with encouraging results, though still limited by acquired resistance. Among the strategies laid out to overcome osimertinib resistance, the use of complex nanoparticles might play a role; a combination of osimertinib and selumetinib (a MEK inhibitor with limited NSCLC effects) conjugated with PEG using a reactive oxygen species-responsive linker had encouraging in vitro and murine model effects. The PEG-selumetinib complex acted as a micelle carrier for osimertinib and delivered the drug payload in high reactive oxygen species activity zones such as tumor cells; such an approach may combine the benefits of both targeting tumor cells and preventing acquired resistance [47][139]. The association of nanotechnologies and EGFR-TKIs is not limited to lung cancer therapy. A creative combination of erlotinib and superparamagnetic iron oxide core particles was found to exhibit affinity towards EGFR overexpressing cells. Such an approach may enable magnetic resonance imaging (MRI)-based detection of EGFR mutated tumors; this would be valuable as MRI techniques are generally of little use for lung imaging despite some obvious advantages, such as no ionizing radiation exposure [48][140]. The anaplastic lymphoma kinase (ALK) gene is located on chromosome 2 and en-codes a transmembrane tyrosine kinase that normally has a low expression in small intestine, nervous system, and testicular cells in adults. Still, ALK gene rearrangement was reported in some NSCLCs; its prevalence is between 3% to 7% in adenocarcinoma cases; many current diagnostic protocols include routine ALK testing for relevant histology samples [49][50][141,142]. The c-ros oncogene 1 (ROS1) codes a tyrosine receptor kinase belonging to the insulin receptor family; some rearrangements have been reported particularly in adenocarcinoma cases occurring in young, never smoking patients (Asian descent may also play a role). Multiple ROS1 mutations have been reported (with various signaling pathways involved). Their global prevalence is estimated between 1 and 3% of lung adenocarcinomas [51][143]. Crizotinib is a tyrosine kinase inhibitor active on ALK, MET and ROS1 available in oral form; its effectivity is limited by various mechanisms such as mutations in the ALK kinase domain, the increased number of ALK fusion genes, and central nervous system progression stemming from low penetration of the blood–brain barrier [52][53][144,145]. Polymeric nanoparticles based on polylactide-tocopheryl polyethylene glycol 1000 succinates (PLA-TPGS) may be used to encapsulate crizotinib with better cellular uptake and increased biological effect [54][146]. Similarly, poly (ethylene glycol)–poly(ε-caprolactones)–poly (ethylene glycol) (PEG–PCL–PEG, PECE) structures have been used as delivery systems for both sorafenib and crizotinib (SORA-CRIZ-NPs), improving their hydrosolubility and reducing their toxic effects [55][147]. Alectinib was approved in 2015 and is included in current therapeutic protocols for ALK-positive NSCLC cases with resistance or progression under crizotinib therapy [56][57][148,149]. Various side effects such as anemia, increased aminotransferase activity, hyperbilirubinemia, and hyperglycemia affect most users [58][150]. One modern oncological approach to lung cancer is the relatively new check-point immunotherapy; this method makes use of immunoglobulins to prevent the interaction between the programmed death ligand-1 (PD-L1) and its receptor (cluster of differentiation 274 (CD274) or PD-1) the underlying mechanism being T-cell cytotoxic mediated [59][60][61][151,152,153]. Silencing PD-L1 and PD-1 on tumor-infiltrating lymphocytes by deploying siRNA on a lipid-coated calcium phosphate carrier proved to be an effective approach in a breast cancer model, suggesting a way to improve immunotherapy outcomes [61][153]. There are some completed clinical trials (Table 13) investigating various nanotechnology therapeutic applications in the field of lung cancer. There is considerable variability in terms of investigative products and efficacy endpoints.Table 13.
Completed clinical trials indexed on
relevant to nanoparticle-augmented lung cancer therapy.
| Study Type | Description | Primary Outcome | NCT Number | Number of Participants |
|---|---|---|---|---|
| Phase IV | Efficacy and safety of paclitaxel liposome and cisplatin compared with gemcitabine and cisplatin as first-line therapy in advanced squamous non-small-cell lung cancer | |||
| TargomiRs (targeted minicells containing a microRNA mimic) as 2nd or 3rd line treatment for patients with recurrent malignant pleural mesothelioma and non-small-cell lung cancer. | ||||
| Establishing maximum tolerated dose and dose-limiting toxicities | ||||
| NCT02369198 | ||||
| 27 | ||||
| Phase II |
Table 24.
Nanoparticle-enabled lung cancer therapeutic procedures.
| Procedure | Nanoparticles | Role | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Progression-free survival | NCT02996214 | 536 | |||||||
| Photothermal therapy | Gold nanoparticles, Fe3O4, polydopamine | [ | 62][63][64][65][154,155, | ||||||
| Phase II | ABI-009, human albumin-bound rapamycin, in patients with metastatic, unresectable, low, or intermediate grade neuroendocrine tumors of the lung or gastro-enteropancreatic system who have progressed or been intolerant to everolimus | Disease control rate | NCT03670030 | 5 | |||||
| Phase II | Safety and efficacy of BIND-014 (docetaxel nanoparticles for injectable suspension) as second-line therapy to patients with non-small-cell lung cancer | Objective response rate | NCT01792479 | ||||||
| Effectiveness of nab-paclitaxel + carboplatin + MPDL3280A (monoclonal antibody directed against the protein ligand programmed cell death-1 ligand 1 (PD-L1) for treatment of non-small-cell lung carcinoma (NSCLC) | |||||||||
| Major pathologic response rate | |||||||||
| NCT02716038 | |||||||||
| 39 | |||||||||
| Fluorescent dye, photosensitizer, theragnostic agent | 156 | , | 157] | ||||||
| Photodynamic therapy | Quantum dots, photosensitizer nanoparticles (hypocrellin B) | Photosensitizer | [66][[69][15867][68],159,160,161] | 64 | |||||
| Phase II | Gene therapyBIND-014 (docetaxel nanoparticles for injectable suspension) as second-line therapy for patients with KRAS positive or squamous cell non-small cell lung cancer | Liposomal nucleic acid delivery system (lipofectamine), solid lipid- and polymer-based gene delivery vectors | Nucleic acid delivery systems | [73][74][75] | Phase I/II | Combination therapy with NC-6004 (nanoparticle-cisplatin) and gemcitabine in patients with advanced solid tumors or non-small-cell lung, biliary, and bladder cancer | Progression-free survival | NCT02240238 | 209 |
| Radiation therapy | ||||||||
| Gold and platinum-based NPs | Sensitizer | [ | 70][71][72][162,163,164] | |||||
| Disease control rate | NCT02283320 | 69 | ||||||
| [ | 165 | , | 166,167] | Phase II | Carboplatin and paclitaxel albumin-stabilized nanoparticle formulation together with radiation therapy and erlotinib in treating patients with Stage III NSCLC that cannot be removed by surgery | |||
| Chemotherapy | Polymers, dendrimers, liposome-based drug delivery systems (various chemotherapeutic agents) | Overall survival at 12 months | NCT00553462 | 78 | ||||
| Carriers, targeted carriers | [ | 18 | ][21][27][28][76][112,115,120,121,168] | Phase II | Paclitaxel albumin-stabilized nanoparticle formulation given together with carboplatin in treating patients with stage IIIB, stage IV, or recurrent NSCLC | Overall response rate | NCT00729612 | 63 |
| Phase I-II | Side effects and optimal dose of ABI-007 (paclitaxel albumin-stabilized nanoparticle formulation) efficacy in treating patients with stage IV NSCLC | Target lesion response (safety, tolerability, antitumor activity) | NCT00077246 | 64 | ||||
| Phase II | CRLX101 (camptothecin (CPT) conjugated to a cyclodextrin-based polymer) vs. best supportive care (BSC) in advanced non-small-cell lung cancer (NSCLC) | Overall survival | NCT01380769 | 157 | ||||
| Phase II | Paclitaxel albumin-stabilized nanoparticle formulation (Abraxane) in treating patients with previously treated advanced non-small-cell lung cancer. | Overall response rate | NCT01620190 | 26 | ||||
| Phase I/II | Safety and antitumor activity of ABI-007 (a unique protein formulation of paclitaxel) in weekly administration in naïve patients with advanced non-small cell lung cancer | Establishing the toxicity | NCT00073723 | 75 | ||||
| Phase I |
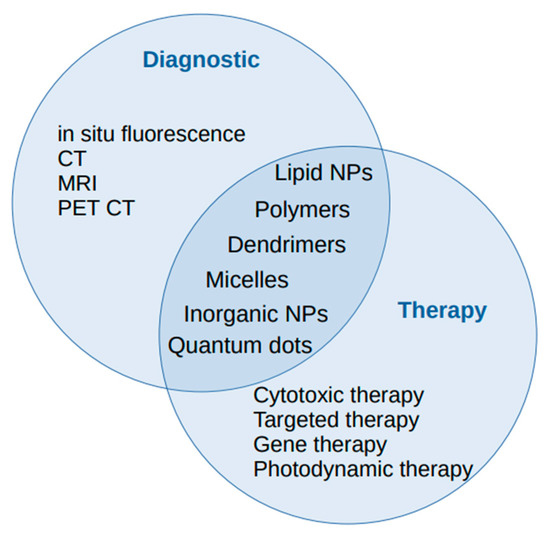
Figure 1.
The diagnostic and therapeutic roles of nanoparticles in lung cancer management.
