Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Lorenzo Iughetti and Version 2 by Sirius Huang.
Central nervous system (CNS) neoplasms are the most common solid tumors diagnosed in children. CNS tumors represent the leading cause of cancer death and cancer-related morbidity for children less than 20 years of age. Neurological, cognitive, and neuropsychological deficits are the most disabling long-term effects of brain tumors in children. Childhood is a time of extreme brain sensitivity and the time of life in which most brain development occurs. Thus, the long-term toxicities that children treated for CNS tumors experience can affect multiple developmental domains and day-to-day functioning, ultimately leading to a poor quality of survival (QoS).
- children
- adolescents
- CNS
- brain tumor
- neurologic late effects
- radiotherapy
- cognitive/neuropsychological outcomes
1. Introduction
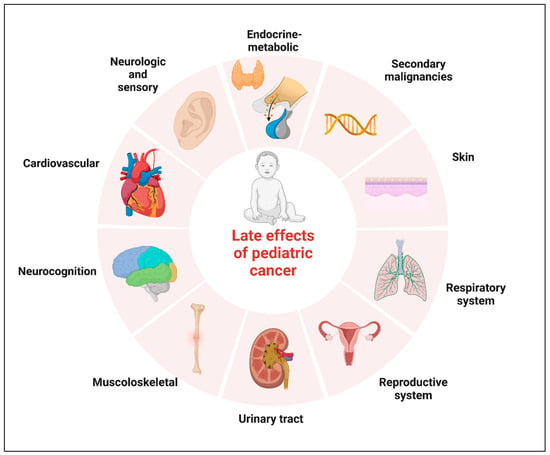
Neoplasms of the central nervous system (CNS) are the most common solid tumors diagnosed in children. Although there has been a moderate increase in survival rates in the last decades, CNS tumors still represent the main cause of cancer-related mortality and morbidity for children less than 20 years of age [1][2][1,2]. Five-year relative survival varies substantially by histological subtype. The relative survival at 5 years now approaches 75% for malignant tumors and 97.9% for non-malignant tumors, according to the data reported in the Central Brain Tumor Registry of the United States (CBTRUS) for the age range 0–19 years [1][3][4][1,3,4]. Significant survival discrepancy by ethnicity still exists for children with CNS tumors, with the largest disparities for diffuse astrocytoma and embryonal tumors [5]. The increase in survival also represents an increasing challenge for oncologists who have to minimize the late effects of tumor diagnosis and treatment. The term “late effects” is broad and applies to complications that begin and can persist after the tumor diagnosis, including lifelong complications. Survivors of childhood cancer can experience many late effects that may affect virtually all organ systems (Figure 1) [6].
The most pervasive long-term effects specific to childhood brain tumors are neurological, neuropsychological, and cognitive dysfunctions [1][7][8][1,7,8]. Cognitive and neuropsychological problems are more frequent in this population compared to survivors of all other pediatric malignancies [3]. It can happen not only because of the experience of a potentially lethal condition and its therapeutic pathway but also due to the involvement of specific cerebral areas, which hits the CNS in a phase of maximum development. Childhood is the time of life during which most CNS development happens; thus, it is not unexpected that CNS tumor survivors commonly experience neurocognitive impairment. A spectrum of disorders ranging from deficits in school functioning to severe dysfunctions can lead to a variable grade of disability, with limitations on everyday activities [3][9][3,9].
Many patients experience both significant global deficits with decreased intelligence quotients (IQ) and specific neuropsychological deficits such as impaired executive functioning, memory, and processing speed [10]. It is estimated that a large proportion of patients treated for a CNS tumor, ranging from 40% to 100%, will experience a deficit in a neurocognitive domain [11][12][11,12]. The simultaneous presence of neurocognitive deficits and neurologic impairments can ultimately lead to a decrease in physical and social functioning and, consequently, to a worsening of patients’ quality of survival [12].
2. Epidemiology of Brain Tumors in Children
Primary CNS tumors represent approximately 25% of pediatric cancers, being the most frequent type of solid tumor in children, and are the main non-traumatic cause of death and disability in the first twenty years of life. The annual average age-specific incidence rate of all malignant and non-malignant CNS neoplasms in childhood and adolescence (0–19 years of age) was about 6.23/100.000 from 2014 to 2018 in the United States [4].
The spectrum of brain tumors in childhood is different in terms of location, histology, and prognosis compared to that in adults, suggesting that the pathogenic events are different [13]. During the first two years of life, supratentorial tumors predominate, whereas infratentorial lesions are more common through the rest of the first decade of life; supratentorial tumors again predominate in late adolescence and adulthood [2]. The most common histology in the younger age, between 0 and 9 years, include gliomas and embryonal tumors; in children of age 10–14 years, gliomas and hypophysis tumors are the most common tumor types. In adolescents between 15 and 19 years of age, pituitary tumors and gliomas again predominate [4][13][4,13].
It is important to remember that CNS tumor classification has long been based on histological morphological characteristics supported by ancillary tissue-based tests (e.g., immunohistochemical, ultrastructural). The 2016 classification introduced molecular markers as key aspects of classification for a relatively small set of entities. Given the large increase in knowledge of the molecular basis of these tumors, the current fifth edition refers to numerous molecular changes that are crucial for the accuracy of the classification of CNS neoplasms [14].
A significant and increasing volume of literature now reports the several long-term effects of brain tumors and their treatment, showing how survival does not come without costs [15]. Pediatric patients with CNS tumors are at high risk of developing physical, neurocognitive, and psychosocial late effects due to tumor site and multimodal therapy combining neurosurgery, chemotherapy, and radiotherapy [15].
3. Neurologic and Sensory Late Effects
Children with brain tumors have malignancies and receive therapy that directly affect the brain, and neurological sequelae are common. Although the majority of brain tumor patients present with acute neurologic deficits that will resolve following treatment, some patients experience persistent deficits [3]. Multiple studies have assessed long-term neurologic and neurosensory deficits [9][16][9,16]. Survivors of childhood brain tumors may suffer debilitating neurologic impairment, with pain, seizures, and sensory loss among the most predominant. The Children Cancer Survivor Study (CCSS) includes a large retrospective cohort of children and adolescents treated for cancer between 1970 and 1986; data collected on adult survivors of CNS malignancies in childhood show that late neurologic conditions are common in this population and increase with time well beyond the 5-year time point. In the CCSS cohort, a cross-sectional study evaluating 5-year survivors of CNS neoplasms demonstrated an increased incidence, compared to siblings, of early- and late-onset neurologic and neurosensory deficits [9]. Seizures were identified in 25% of patients, more frequently associated with supratentorial tumors [9]. Among the population of survivors without previous seizures in the first 5 years after diagnosis, 33% would experience new onset seizures beyond the 5-year period. Similarly, a high percentage of patients would report visual deficits, with a lower percentage of about 3% for cataracts to a higher proportion of patients complaining of diplopia (17%) [16]. Phillips et al. suggest that pediatric CNS cancer survivors experience additional neurocognitive risk if they develop a seizure diagnosis, with seizure resolution associated with improved attention and memory [17]. In a large cohort of adults survivors of pediatric CNS tumors treated at St. Jude Children’s Research Hospital, the survivors with a history of seizures were at risk of severely impaired academics, attention, and memory dysfunction compared with those without a history of seizures, even after adjustment for cranial radiotherapy (CRT) exposure [18].
Hearing loss is of particular importance in that the cochlea is highly sensitive to toxicity from radiation and platinum-based chemotherapy; sensorineural hearing loss is associated with worse cognitive and functional outcomes, and intact hearing is critical for language development [19][20][19,20] Twelve percent of patients in the CCSS cohort report hearing impairment with a statistically significant relationship to posterior fossa irradiation greater than 50 Gy [9]. Ototoxicity is most commonly a result of treatment with platinum analogs that cause direct cochlear damage, possibly due to reactive oxygen species-induced cellular destruction [21][22][21,22]. Risk of ototoxicity is compounded with cranial radiation therapy treatment, with doses above 32 Gy increasing the risk of ototoxicity in a dose-dependent manner [23][24][23,24]. More recent studies [23] prospectively examined hearing impairment and associated risk factors and found that RT is independently associated with neurosensorial hearing loss even when ototoxic chemotherapy is not administered; Bass et al. also reported children younger than 3 years at RT initiation, with CSF shunt and receiving higher doses to be at higher risk [23]. Brain tumors can also alter the normal neuroanatomical structures of the visual system leading to visual impairment and dysfunction. Visual impairment in childhood is associated with lifelong effects on self-perception, childhood development, and quality of life [25]. Peripheral neuropathy is a common side effect of platinum analogs and vinca alkaloids; these neuropathic symptoms usually resolve following treatment, but, in some cases, they persist for years after therapy [26].
The CCSS cohort demonstrates cumulative incidence of seizures, motor impairment, and hearing loss increases from 5 to 30 years post-diagnosis [27]. Pediatric CNS cancer survivors, unfortunately, are also at increased risk for stroke, which drastically worsens after exposure to CRT [1].
4. Cognitive and Neuropsychological Outcomes
Cancer-related cognitive dysfunction affects about one-third of childhood cancer survivors in the US [28]. Neurocognitive late effects in childhood brain tumor survivors are relatively common and play a significant role in modifying Health-related Quality of Life (HR-QOL). Traditionally, the measurement of neurocognitive function has been conducted through the assessment of intellectual quotient (IQ). Declines in IQ are evident in pediatric CNS tumor survivors as early as the first year following diagnosis and treatment, with potential progression over the next 5 to 7 years [29]. Children treated for medulloblastoma are reported to have a progressive decrease in their IQ over time, losing 2–4 points per year over 7 years from the time of diagnosis [30][31][30,31]. However, more recently, researchers have focused on neuropsychological functions and have identified the ones at greatest risk, believed to represent core deficits that contribute to broader difficulties [3][29][30][3,29,30]. The most frequently impaired functions include attention, working memory, and processing speed [3][30][32][3,30,32].
Executive functions (EFs) deficits are common and are primarily associated with cerebellum-cerebral pathway dysfunction.
Speech and language deficits are documented among nearly all survivors after post-surgery posterior fossa syndrome [15][32][15,32].
For some authors, all these functional impairments are thought to be the mechanism behind the decline in IQ. It is reasonable to hypothesize that the neurocognitive decline seen in CNS tumor survivors may be due not predominantly to loss of learned information but rather to a failure in achieving age-related gains in cognitive function [3].
4.1. Attention, Working Memory, and Processing Speed
Processing speed is commonly defined as the promptness in performing relatively automatic mental tasks. Some authors report this domain as the first to be impaired after treatment and to be specifically correlated with craniospinal irradiation (CSI) dose [30]. Brinkman et al., however, in a large cohort of adult survivors of pediatric CNS tumors, report a consistent group of 37% of survivors without CRT exposure but severely impaired on at least one measure of processing speed [18]. Given the importance of myelin in the conduction speed of neural impulses, it is not surprising that deficit in this domain is frequent in children with cancer involving the CNS [18].
Working memory is a temporary workspace in which information is stored and handled for a brief period of time; working memory tasks have been shown to activate the prefrontal cortex [32]. Several studies have reported specific deficits in working memory in the population of children treated for posterior fossa tumors. In particular, a working memory deficit can emerge very early with a progressive decline over time [30]. Edelstein et al. showed that working memory was the only domain with a continuous decline over time despite a stable IQ score 20–40 years after diagnosis of posterior fossa tumors [33].
Attention is the ability to remain alert or focused. It is not a unitary construct, and the subset of skills that falls under the concept of attention includes sustained, focused, selective, divided, alternating, shifting, and resistance to distraction. Attention is demanded by many different tasks, and conclusions about attention functioning can be drawn using subtests of various test batteries [34]. Attention deficits are commonly found in pediatric patients treated for CNS tumors and are reported to appear later than processing speed decline [30]. However, attentional and mnemonic deficits are reported to be present early at diagnosis by some authors [35].
These neuropsychological functions are essential for competence and knowledge acquisition, and their dysfunction is considered to be the core deficit explaining academic underachievement [32].
4.2. Executive Functions
EFs are different but closely interacting skills required for efficient and appropriate behavior. They are often identified as the ability to control, organize and manage cognitive, emotional, and behavioral responses. These functions rely on the integrity of multiple cooperating brain networks [36]. EFs involve inhibition, mental flexibility, planning and decision-making, abstract reasoning, concept formation, problem-solving, and awareness [37]. There has been some debate about whether it is appropriate to assess executive functioning in children, given that the prefrontal cortex is the last cortical area to reach maturation. In contrast, current research has provided evidence for the development of EF in children and for deficits in these areas following brain injury related to normal development. Therefore, it is important to include measures of EF when evaluating children with cancer involving CNS. Studies on EFs in pediatric CNS tumors have reported these patients to be particularly susceptible to EFs impairments, both in the short and long term. Pediatric cerebellar tumor survivors exhibit similar patterns of impairment in executive functions, in particular in forward-thinking, mental flexibility, and inhibition [38].
Deficits in this area can manifest in daily life as problems with organization, time management, and emotional and behavioral control and will also negatively impact social development [37]. Optimal executive functioning is reported to play a crucial role in long-term functional outcomes. In the context of intact global intellectual functioning, communication, and memory skills, impairments in EFs can cause the greatest handicap for social attainment and adaptive functioning [36] (Table 1).
Table 1. Cognitive domains impacted by tumor and its treatment and studies describing characteristics and risk factors of impairment.
| Cognitive Domain | Author, Year | Population | Main Findings |
|---|---|---|---|
| Global intellectual functioning |
De Ruiter et al. [38] | Meta-analysis summarizing neurocognitive outcomes of 1082 pediatric brain tumor survivors | PBTS scored on average 0.54SD to 0.90SD lower on the WISC-III scales than the normative sample, with PIQ scores being even more depressed than VIQ scores |
| Brinkman et al. [17] | 224 adult survivors of CNS pediatric tumors | 20–30% of survivors demonstrated impairment on performance-based measures of intellect compared to expected 2% in the general population | |
| Lafay-Cousin et al. [39] | 16 Atypical teratoid/rhabdoid tumor survivors | Overall impaired neurocognitive outcome while treated with a radiation sparing approach | |
| Clark et al. [40] | 43 survivors of focal low-grade brainstem gliomas | Measures of intelligence quotient significantly lower than normative, despite focal disease and treatment targeting subcortical areas | |
| Moxon-Emre et al. [41] | 113 patients treated for medulloblastoma | Patients treated with reduced dose craniospinal irradiation plus tumor bed boost showed stable intellectual trajectories while those treated with higher doses and larger boost experienced decline. | |
| Roncadin et al. [42] | 29 astrocytoma and 29 medulloblastoma survivors | Greater perioperative and short-term medical adversity contributes to lower IQ in the long term | |
| Margelisch et al. [35] | 20 CNS tumor patients compared to 27 control patients (other type of cancer) at diagnosis | Mean IQs of patients with brain tumor lie within the normal range at diagnosis | |
| Executive functions | Law et al. [43] | 25 children treated for medulloblastoma with surgery, CRT and chemotherapy and 20 healthy controls | EFs deficits are found children treated for medulloblastoma compared to age-matched peers. Selective deficits in cognitive efficiency, problem-solving and working memory. Specific damage to cerebrocerebellar circuitry. |
| Heitzer et al. [44] | 32 patients treated for LGG with surgery only | Supratentorial LGG and history of seizures: greater impact on executive functioning |
|
| Koustenis et al. [45] | 42 pediatric posterior fossa tumor survivors (mean age 14.63 years | Pediatric cerebellar tumor survivors show similar pattern of impairment in executive functions in particular in forward-thinking, mental flexibility and inhibition | |
| Memory | Margelisch et al. [35] | 25 children treated for medulloblastoma with surgery, CRT, and chemotherapy and 20 healthy controls | Memory and attention are the principal domain found to be impaired at diagnosis before treatment |
| Decker et al. [46] | 29 PBTS treated with chemotherapy and CRT | Associations between hippocampal subfield volumes and short-term verbal memory | |
| Attention | Margelisch et al. [35] | 25 children treated for medulloblastoma with surgery, CRT, and chemotherapy and 20 healthy controls | Memory and attention are the principal domain found to be impaired at diagnosis before treatment |
| Processing speed | Weusthof et al. [47] | 103 CNS pediatric patients treated with photon therapy, proton therapy or surgery alone | Processing speed is the most vulnerable domain with decline over time in both photon and surgery cohorts |
| King et al. [48] | 57 neurotypical controls and 57 survivors of childhood brain tumors | Processing speed appears to be the central cognitive skill that disrupts the other core cognitive skills of attention span and working memory, and all three make a unique contribution to IQ and academic achievement |
PBTS: pediatric brain tumor survivors; PIQ: performance intelligence quotient; VIQ: verbal intelligence quotient.