Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Patrizia Limonta and Version 2 by Rita Xu.
Prostate cancer (PCa) is the second leading cause of cancer deaths among men in Western countries. Mitochondria, the “powerhouse” of cells, undergo distinctive metabolic and structural dynamics in different types of cancer. PCa cells experience peculiar metabolic changes during their progression from normal epithelial cells to early-stage and, progressively, to late-stage cancer cells.
- prostate cancer
- mitochondrial metabolism
- mitochondrial dynamics
1. Introduction
Mitochondria are organelles involved in different cellular processes, including cell proliferation and intrinsic apoptosis, redox and Ca2+ homeostasis as well as cell stemness. They are also known as the master producers of ATP, being deeply involved in cellular energy metabolism; in addition, being dynamic organelles, they often undergo structural changes, including biogenesis, fusion/fission and mitophagy. Specifically, it is now well recognized that mitochondria undergo complex functional and structural dynamics in cancer cells during the different phases of tumor growth and progression.
1.1. Mitochondrial Metabolism in Cancer Cells
It is now recognized that cancer cells, growing in a hypoxic and hyponutrient microenvironment, are forced to adapt their metabolism (“metabolic reprogramming”) to obtain the required amount of biomass and energy to sustain their uncontrolled proliferation and aggressive behavior.
According to the theory proposed by Otto Warburg in the 1920s (named the Warburg effect), cancer cells are characterized by high rates of glucose uptake and preferentially metabolize it through the glycolytic pathway, even in the presence of adequate amounts of oxygen (aerobic glycolysis) and functional mitochondria [1][2][1,2]. Although a small amount of ATP per mole of glucose is produced through glycolysis, it is believed that this metabolic process rapidly produces high levels of metabolites to sustain the biosynthesis of the molecules (i.e., amino acids, fatty acids and nucleotides) required for cancer cell growth and division [3][4][5][6][7][8][3,4,5,6,7,8]. Moreover, the high levels of lactate produced at the end of glycolysis by lactate dehydrogenase (LDH) are secreted by cancer cells to generate an acidic tumor microenvironment promoting their transition to the most aggressive (i.e., migratory, invasive) phenotype and affecting the immune microenvironment to induce an immune tolerant condition [9][10][9,10].
Despite the presence of an active glycolytic pathway, several recent data strongly support the coexistence of functional mitochondria in cancer cells, even in the metastatic phase [6][11][12][13][14][15][16][17][18][19][20][6,11,12,13,14,15,16,17,18,19,20]. Mitochondria, known as the “powerhouse of the cell”, are deeply involved in the cellular metabolic dynamics, being the major intracellular producers of ATP through the oxidative phosphorylation (OXPHOS) pathway; these organelles are also the “venue” where the tricarboxylic acid (TCA) cycle takes place to provide the most building blocks for the synthesis of biomolecules. A high level of fatty acid β-oxidation also occurs in mitochondria to sustain the production of citrate. Moreover, glutaminolysis is activated to convert glutamine into intermediates for the synthesis of amino acids and nucleotides, as well as into glutamate to fuel the TCA cycle. Together, these metabolic pathways are necessary for cell anabolism to trigger cell proliferation and metastasis in cancers [8][20][21][22][23][24][25][26][27][28][29][30][8,20,21,22,23,24,25,26,27,28,29,30].
A metabolic remodeling based on a shift towards the OXPHOS machinery has also been shown to be involved in the development of drug resistance in tumor cells [31][32][33][34][31,32,33,34] and to occur in the subpopulation of cancer stem cells (CSCs), known to play a key role in tumor relapse, to provide sufficient amounts of energy and metabolites for their self-renewal and evasion from cell death induced by anticancer drugs [35][36][37][38][39][40][41][42][43][35,36,37,38,39,40,41,42,43].
Cancer cell metabolic plasticity has also been reported to be regulated by neighboring cells in the tumor microenvironment through both mechanical and chemical factors [18][44][18,44]. For instance, it has been shown that cancer-associated fibroblasts (CAFs) secrete lactate that is taken up by tumor cells to trigger their metabolic switch towards the OXPHOS energy-producing pathway and reactive oxygen species (ROS) generation [45][46][47][48][45,46,47,48].
The key role played by mitochondrial metabolism in cancer is strongly supported by the observation that different compounds were shown to exert their anticancer activity by targeting the oxidative phosphorylation pathways [42][49][50][51][52][53][54][55][56][42,49,50,51,52,53,54,55,56].
1.2. Mitochondrial Dynamics in Cancer Cells
Mitochondria are also highly dynamic organelles undergoing changes in number and structure through different processes such as biogenesis, fusion, fission (fragmentation) and mitophagy (removal of impaired mitochondria). A balance between these processes is required for the maintenance of homeostasis in healthy cells; on the other hand, adaptations of mitochondrial dynamics have been widely reported in cells undergoing metabolic and stressful conditions (i.e., glucose starvation, hypoxia), such as cancer cells [8][57][58][59][60][61][62][8,77,78,79,80,81,82].
Mitochondrial biogenesis is the generation of new organelles from pre-existing ones. In cancer cells, an increase in mitochondrial mass is induced by a variety of stressful signals and has been found to correlate with cell growth, invasiveness, metastasis and drug resistance [63][64][83,84]. The master regulator of mitochondrial biogenesis is peroxisome proliferator-activated receptor gamma coactivator 1α (PGC1α) which cooperates with different transcription factors to increase the expression of the mitochondrial transcription factor (TFAM), the final effector of the increase of mitochondrial mass [65][85]. PGC1α, activated by phosphorylation by the energy sensor adenosine monophosphate-activated protein kinase (AMPK) and by deacetylation by silent information regulator 1 (SIRT1), also triggers the transcription of both nuclear and mitochondrial genes, leading to increased mitochondrial mass, OXPHOS activity and ATP production [66][67][68][86,87,88]. Mitochondrial biogenesis has been shown to mediate the ability of CSCs to overcome antitumor therapies [69][70][71][89,90,91].
An imbalance in the mitochondrial fusion/fission leads to peculiar changes of the morphological features of these organelles (interconnected vs. fragmented). GTPases belonging to the dynamin family play a pivotal role in mediating both of these processes [16][59][62][72][16,60,79,82]. Mitochondrial fusion is the physical merging of the outer membranes (OMM) and the inner membranes (IMM) of distinct mitochondria and depends on GTP hydrolysis. This process foresees the activity of three GTPases, mitofusin (MFN) 1 and 2 on the OMM and optic atrophy protein 1 (OPA1) on the IMM. MFN 1 and 2 interact to induce the formation of strict connections between adjacent mitochondria, leading to the fusion of the OMM. Then, OPA1 interacts with MFNs forming intermembrane protein complexes, thus coupling the fusion of OMMs with IMMs [73][74][75][76][92,93,94,95]. Mitochondrial fission is a multi-step process allowing the division of one mitochondrion, leading to the formation of new organelles. The key protein involved in this process is dynamin-related protein 1 (DRP1), a cytosolic GTPase. Endoplasmic reticulum (ER) membranes get in touch with mitochondria allowing a Ca2+ flux from the ER into the mitochondria, thus favoring actin polymerization and inner mitochondrial membrane constriction at this site. At the level of ER-mitochondria contact sites, different proteins such as mitochondrial dynamics 49 and 51 (MID49 and MID51), MFF and mitochondrial fission 1 (FIS1) proteins, identified as DRP1 receptors, are also located. DRP1 binds to these proteins to encircle and shrink the mitochondria, finally leading to their fission. Accumulating evidence demonstrates that an imbalance in these mitochondrial dynamics occurs in different types of cancer [59][77][78][79][80][79,96,97,98,99].
2. Mitochondrial Metabolism and Dynamics in PCa
Prostate cancer (PCa) represents the most common malignancy among men and the second leading cause of cancer deaths in developed countries [81][107]. Androgen-deprivation therapy (ADT), based on gonadotropin-releasing hormone (GnRH) agonists and antagonists, either alone or in combination with androgen receptor antagonists (enzalutamide, apalutamide, darolutamide), still remains the most common treatment for androgen-dependent PCa patients [82][83][84][85][86][87][88][108,109,110,111,112,113,114]. However, PCa often progresses towards the castration-resistant phase (CRPC), a condition characterized by the acquisition of a more aggressive and metastatic behavior even in the absence of circulating androgens [89][90][115,116]. The standard treatment of CRPC patients is presently based on chemotherapy (docetaxel) either alone or in combination with GnRH analogs, antiandrogens or inhibitors of androgen synthesis (abiraterone) [91][92][93][94][95][96][97][98][99][100][101][117,118,119,120,121,122,123,124,125,126,127]. Novel therapeutic strategies, such as immune check-point inhibitors or CAR-T approaches, are under investigation [102][103][104][105][128,129,130,131]. The elucidation of novel molecular hallmarks of tumor progression (i.e., mitochondrial metabolism and dynamics) will likely pave the way for the development of novel therapeutic approaches for PCa patients. Based on the above considerations, as well as on the progressively accumulating data in the literature, in this researchersview we aim to highlight and discuss the recent findings on the involvement of mitochondrial functional reprogramming and structural dynamics in PCa, specifically in its CRPC stage; researcherswe also address the impact of these mechanisms as molecular targets of novel and effective antitumor strategies for this aggressive disease.
2.1. Mitochondrial Metabolism
Metabolic reprogramming is a well-recognized hallmark of cancer, enabling cancer cells to acquire properties that support cell survival, proliferation and acquisition of aggressive (invasive, metastatic) features. However, peculiar molecular mechanisms of this metabolic rewiring have been reported to occur in different types of tumor cells, and this metabolic heterogeneity confers differences in their proliferative/metastatic potential. In prostate epithelial cells, distinctive changes of cell metabolism have been highlighted during the different phases of their conversion from healthy cells to early-stage and, progressively, to late-stage cancer cells [31][106][107][108][109][31,132,133,134,135].
2.1.1. Metabolic Rewiring
Healthy epithelial cells, located in the peripheral zone of the prostate, exhibit a peculiar metabolic programming aimed at producing and secreting citrate into the prostatic fluid, one of the most relevant functions of this gland [110][136]. In most mammalian cells, pyruvate, the end product of the glycolytic pathway, is transported into the mitochondria where it is decarboxylated to acetyl-CoA. Acetyl-CoA subsequently binds to oxaloacetic acid to form citrate that enters the TCA cycle. Citrate is then converted into its isomer isocitrate that is further oxidized into the TCA cycle for the progression to OXPHOS and ATP production; mitochondrial aconitase (m-aconitase) is the ROS-sensitive key enzyme responsible for the citrate-isocitrate conversion. In normal prostate epithelial cells, m-aconitase activity is inhibited, resulting in the impairment of citrate oxidation followed by its accumulation and secretion [111][137]. The inhibition of m-aconitase strictly correlates with the ability of these cells to accumulate zinc, due to their elevated expression of its transporter ZIP1; high intramitochondrial zinc levels increase ROS generation, leading to the inhibition of m-aconitase activity and resulting in a truncated TCA cycle [112][138]. As a consequence, the healthy “zinc-accumulating, citrate-producing” epithelial cells are characterized by an inefficient OXPHOS which is compensated by an increased glycolytic pathway to support citrate production [113][139]. High zinc levels were also found to be associated with a mitochondrial apoptotic phenotype mediated by the release of cytochrome c and caspase activation [114][140].
On other hand, it is now well established that prostate epithelial cells undergo a peculiar metabolic rewiring during the early phases of cancer development. Specifically, elevated levels of the TCA cycle enzymes and intermediate metabolites could be detected in prostate cancer tissues in comparison to adjacent normal tissues [115][116][117][141,142,143]. Intracellular zinc levels were found to be significantly reduced in PCa cells, thus leading to the reactivation of m-aconitase, citrate oxidation, TCA cycle pathways and oxidative phosphorylation [118][144]. This reduction was shown to be related to a decreased expression of zinc transporters, such as ZIP1 and 3 [119][145], mediated by the hypermethylation of their gene promoters [120][146]. The low levels of zinc also allow cancer cells to avoid apoptosis; actually, it has been reported that zinc treatments trigger cell death and promote chemosensitivity in PCa cells [121][147]. In line with these observations, very low zinc levels were observed in PCa tissues [122][148]. Taken together, these data support that the transformation of prostate epithelial cells into their tumoral phenotype is associated with an efficient reactivation of the TCA cycle/OXPHOS metabolic pathway to meet their increased energy and metabolite demand [123][149].
Interestingly, prostate epithelial cells seem to possess a markedly metabolic plasticity by changing their mitochondrial metabolic features again when progressing from the early-stage towards the late-stage (i.e., metastasis) of cancer, even in the presence of low intracellular zinc levels. Specifically, the Warburg effect (i.e., increased glycolytic activity) has been proposed as the prominent metabolic feature of metastatic prostate tumors [123][124][149,150]. Mechanistically, the PI3K-AKT-mammalian target of the rapamycin (mTOR) pathway, a key driver of tumor progression, was shown to play a causal role in prostate tumorigenesis through the up-regulation of pyruvate kinase isoenzyme type M2 (PKM2), the rate-limiting enzyme catalyzing the final reaction of the glycolytic pathway [125][126][151,152]. Mutations of the tumor suppressor p53, frequently occurring in advanced prostate cancers, were reported to trigger the Warburg effect. Moreover, deletions of the tumor suppressor PTEN, often observed in aggressive prostate tumors, were demonstrated to correlate with an increased expression of hexokinase 2 (HK2), the initial enzyme of glycolysis, catalyzing the phosphorylation of glucose by ATP to glucose-6-P through the AKT-mTOR pathway [123][127][149,153]. Taken together, these observations support that PTEN and p53 tumor suppressors, together with the PI3K-AKT-mTOR pathway, are essential drivers of the Warburg effect to maintain a sufficient energy and metabolites supply for PCa growth and progression [128][154]. In addition, it has been demonstrated that, in prostatic carcinoma cell lines, the hypoxic conditions of the tumor microenvironment trigger the expression of SUMO1/sentrin-specific peptidase 1 (SENP1) that in turn interacts with HIF1α to promote the Warburg effect and sustain cell proliferation [129][155]. In line with these data, Sun and coworkers recently reported that HK2 and HIF1α are highly expressed in PCa tissues and their expression correlates with tumor growth and metastasis [130][156].
There is also consistent evidence that an association exists between obesity and the risk of PCa growth [131][157]. A deleterious bidirectional cross-talk between PCa cells and adipocytes in their microenvironment has been demonstrated [132][158]. Specifically, it has been reported that PCa cells educate neighboring adipocytes towards a lipolytic phenotype, resulting in free glycerol production and secretion; adipocyte-derived glycerol is then uptaken by PCa cells to enter and fuel the glycolytic pathway [133][159]. Moreover, adipocyte conditioning of PCa cells leads to an increased expression of glycolytic genes, resulting in lactate production and OXPHOS inhibition [133][134][159,160]. In line with these observations, rwesearchers recently reported that adipocyte-released extracellular vesicles significantly decrease the sensitivity of PCa cells to the chemotherapeutic drug docetaxel and this effect is associated with an AKT/HIF-1α axis-related Warburg effect, which is characterized by enhanced glucose consumption, lactate release and ATP production [135][161].
To confirm that PCa cells undergo dynamic metabolic changes at each stage of tumor development, Vayalil and Landar introduced the “mitochondrial oncobioenergetic index (MOBI)” (i.e., the mathematical representation of the oncobioenergetic features of a tumor cell). In PCa cells with progressive malignant behaviors, they demonstrated that MOBI values (representative of OXPHOS activity) are high in premalignant prostate cells and significantly decrease with increasing malignancy [107][133].
Taken together, these observations support that PCa cells reprogram their metabolism towards the aerobic glycolysis (the Warburg effect) in the context of tumor progression. However, contrasting results, demonstrating that the high levels of OXPHOS activity observed in primary tumors still persists during the progression of the pathology towards its metastatic stage, have also been reported in the literature [136][137][138][139][162,163,164,165].
Galbraith and coworkers recently demonstrated an association between peroxisome proliferator-activated receptor gamma (PPARG) expression and metastatic features in PCa. By means of in vitro and in vivo studies, these authors could show that, in PCa cells, PPARG overexpression induces AKT3 expression leading to increased mitochondrial biogenesis and ATP production, finally fueling tumor cell epithelial-to-mesenchymal transition (EMT) and metastatic behavior [140][166]. Pyruvate dehydrogenase complex (PDC) is the multi-protein complex that catalyzes the conversion of pyruvate to acetyl-CoA, thus fostering mitochondrial activity. It was reported that, in PCa cells, knockout of the major subunit of PDC (PDHA1) is accompanied by lower levels of the TCA cycle activity, resulting in impaired OXPHOS activity and growth of these cells when xenografted in nude mice [137][163]. Pyruvate kinase isozyme 2 (PKM2) has been shown to be highly expressed in many types of cancer cells, including PCa cells. Interestingly, in these cells, PKM2 has been reported to be also deeply involved in glucose metabolism (OXPHOS activity) and to mediate proliferation, metastatic behavior and acquisition of stem cell properties [141][142][143][167,168,169]. Mitochondrial pyruvate carrier (MPC) is the hetero-dimeric complex (formed by MPC1 and MPC2) responsible for the import of pyruvate into the mitochondria where it is converted to acetyl-CoA and then further enters the TCA cycle to fuel the OXPHOS machinery. MPC2 expression was found to correlate with tumor aggressiveness in PCa specimens [144][145][170,171]. Transcriptional enhanced associate domain 4 (TEAD4) is a transcription factor previously shown to be involved in the regulation of the expression of mitochondrial genes involved in the OXPHOS pathways [146][172]. TEAD4 is expressed in PCa cells, and its expression has been reported to be critical in increasing OXPHOS activity. In a recent paper, Chen and coworkers reported that TEAD4 expression is epigenetically regulated by the semi-essential amino acid arginine to modulate OXPHOS functions in hormone-refractory PCa cells [147][173].
Interestingly, elevated OXPHOS and mitochondrial mass have been observed in the aggressive stem cell subpopulation of different tumors, including PCa [148][174]. Sotgia and coworkers proposed the development of a “mitochondrial based oncology platform” for specifically targeting CSC metabolism [149][150][175,176]. In line with this observation, metformin has been proposed as an effective anticancer agent based on its ability to specifically target OXPHOS and ATP production in prostate CSCs [151][177]. On the other hand, impaired mitochondrial OXPHOS and upregulated glycolysis were observed in these cells [152][178]. Thus, the presence of exacerbated OXPHOS in PCa stem cells still remains a controversial issue.
Given the crucial role of the tumor-stroma cross-talk in shaping cancer cell metabolism, Ippolito et al. investigated how CAFs might regulate mitochondrial dynamics in PCa cells. They found that tumor-associated CAFs significantly enhance mitochondrial respiration, mediated by a lactate shuttle, and favor mitochondria transfer in PCa cells, thus promoting their malignant behavior [46]. In line with these results, Grupp et al. demonstrated that a high mitochondria content in PCa specimens correlates positively with PCa progression and represents an effective predictor of a poor clinical prognosis and outcome [106][132]. Last but not least, a switch from glycolysis to OXPHOS activity has been observed in PCa cells undergoing the development of resistance to standard therapies (i.e., enzalutamide, docetaxel) [153][179].
Based on these observations, it is now accepted that targeting both glycolysis and mitochondrial OXPHOS pathway might represent an effective therapeutic strategy for advanced, metastatic and drug-resistant PCa [154][180].
A schematic overview of the metabolic rewiring occurring in prostate epithelial cells during the different stages of cancer progression is given in Figure 1.
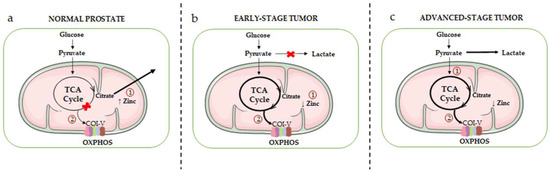
Figure 1. Schematic overview of the mitochondrial metabolic rewiring occurring in prostate epithelial cells during the different stages of cancer progression. (a) Healthy prostate epithelial cells accumulate high levels of zinc (due to the overexpression of its transporter ZIP1), resulting in the inhibition of mitochondrial m-aconitase, the key enzyme responsible for the citrate-isocitrate conversion in the TCA cycle (1). This inhibition ultimately leads to the truncation of the TCA cycle and to citrate accumulation and secretion. As a result, normal prostate epithelial cells are characterized by an inefficient OXPHOS machinery (complexes I-V, COI-V) (2). (b) In the early stage of tumor progression, intracellular zinc levels are significantly reduced (due to a decreased expression of its transporter) (1); this leads to the reactivation of m-aconitase, restoring the citrate-isocitrate conversion, and consequently of the TCA cycle and OXPHOS metabolic pathways (2). (c) In the advanced stage of tumor progression, PCa cells exhibit the Warburg effect, an active aerobic glycolysis accompanied by high levels of lactate production (1). However, it is now well established that a high activity of the TCA cycle/OXPHOS pathways still persists in these cells, and it is even exacerbated in PCa stem cells as well as in drug-resistant PCa cells (2).
2.1.2. The AR-Mitochondria Axis
ADT still represents the therapy of choice for early-stage, androgen-dependent PCa. However, most patients progress towards the aggressive CRPC stage characterized by a high rate of cell proliferation, invasiveness and metastatic behavior; interestingly, in most CRPC patients (about 80%) a reactivation of the androgen receptor (AR) has been observed. The persistent activity of AR in this stage has been shown to involve different mechanisms, including AR gene amplification, AR mutations, AR gene alternative splicing, generating different receptor splice variants and intratumoral synthesis of androgens. Since PCa progression is also associated with a peculiar metabolic reprogramming, as discussed above, it has been postulated that AR might be a master regulator of PCa cell metabolism. In line with this hypothesis, genomic, transcriptomic and metabolomic functional studies pointed out that AR regulates different metabolic pathways, including glucose uptake (through the induction of different glucose transporters), glycolysis, TCA cycle, mitochondrial biogenesis and respiration, de novo lipid synthesis and fatty acid β-oxidation [117][144][155][156][157][158][143,170,181,182,183,184].
Different intracellular signaling pathways were reported to be involved in this AR-driven metabolic reprogramming. Importantly, AR activation was demonstrated to induce mTOR translocation into the nucleus where it binds to the promoter regions of metabolic genes (such as HK2), thereby regulating their expression; accordingly, inhibition of the mTOR pathway resulted in impaired glycolytic activities and reduced proliferation in PCa cells [157][183]. In AR-expressing PCa cells, it was shown that androgens promote the activity of the AMPK/PGC1α signaling cascade, leading to increased glycolytic rates, mitochondrial biogenesis, OXPHOS, intracellular ATP levels and cell growth [156][182].
An additional interesting mediator of the AR metabolic activity is MPC (specifically the MPC2 isoform), reported to be highly expressed in AR-positive PCa cells (both hormone-dependent and CRPC cells) but almost absent in AR-negative PCa cells and to play a pivotal role in supporting a functional TCA cycle. Bader and coworkers demonstrated that MPC is transcriptionally upregulated by AR in PCa cells, and its inhibition impairs O2 consumption, TCA cycle metabolite levels and oxidative phosphorylation, thus halting cell proliferation. Moreover, these authors could show that targeting MPC with the MPC inhibitors UK5099 and MSDC0160 (this inhibitor is orally administered and clinically viable) results in the suppression of the growth of AR-expressing, but not AR-negative, PCa cells in in vitro and in vivo studies [117][144][159][143,170,185]. In line with these results, it has been reported that, in androgen-sensitive and CRPC cells, activation of the AR signaling upregulates the expression of DRP1 (the mediator of the mitochondrial fission) to induce the formation of the VDAC/MPC2 complex and thereby the pyruvate transport into the mitochondria and sustains mitochondrial metabolic pathways, such as OXPHOS.
2.1.3. mtDNA Mutations
So far, most studies addressing the relevance of genetic mutations in PCa development have been focused on the nuclear genome. Mitochondria, maternally inherited organelles, are deeply involved in the process of tumorigenesis by orchestrating metabolic and energy production pathways, ROS signaling and apoptosis [8][160][161][8,57,189]. Thus, dissecting the mitochondrial genome is currently considered an essential step to obtain a complete view of the genetic alteration profile in PCas.
The majority of the proteins of the four ETC complexes (COI-IV) involved in OXPHOS are encoded by nuclear DNA; however, 13 proteins in these complexes are encoded by mtDNA, the small circular DNA molecule found inside mitochondria. The mtDNA is characterized by a high mutation rate, mainly linked to high levels of ETC-derived ROS and a low efficient DNA repair system in these organelles [162][190]. Mutations of mtDNA have been found in different types of human cancers, including PCa [136][163][164][165][166][167][162,191,192,193,194,195], although their functional role still needs to be fully elucidated. Gomez-Zaera and coworkers analyzed the presence of mtDNA sequence variants in human PCa tissues; they reported that the most frequent variants were present in the following genes: mt-RNR2, encoding the large 16S mitochondrial ribosomal RNA (rRNA) subunit; mt-D-loop (displacement loop, control sites for the expression of the mitochondrial genome); and mt-ND4, encoding the protein NADH dehydrogenase 4, part of the COI of the ETC pathway [168][196].
The analysis of somatic mutations in tumor tissues from PCa patients pointed out their presence in genes coding for rRNA (mt-RNR1 and mt-RNR2), transfer RNA (tRNA) and the protein-coding gene mitochondrially encoded ATP synthase membrane subunit 6 (mt-ATP6), that encodes the ATP synthase Fo subunit 6 (or subunit/chain A). Moreover, somatic mutations in the entire mitochondrial genome were found to be associated with high PSA levels in PCa patients [169][197]. By using a yeast model organism, it was shown that the mutation mt-ATP6-P136S specifically found in PCa tissues positively correlates with tumor progression and may be involved in cancer cell escape from apoptosis [170][198]. Hopkins et al. reported that mutations in mitochondrial rRNA, tRNA as well as in the protein-coding genes mt-ATP6, mt-ND1, mt-ND2 and mitochondrially encoded cytochrome c oxidase I (mt-CO1), component of the complex IV, cytochrome c oxidase, the last enzyme in the mitochondrial electron transport chain which drives OXPHOS, are frequent in PCa tissues and are drivers of PCa aggressive behavior [167][195]. In line with these observations, mutations in genes encoding for proteins of the mitochondrial complex I (mt-NDs) were reported to be frequent in high grade PCa tissues and to be associated with a reduced activity of the NADH dehydrogenase pathway and an increased, compensatory, activity of the succinate-using FADH2 pathway [171][199]. Interestingly, Sun and coworkers demonstrated that the presence of a mutant mt-CO1 gene results in the resistance of PCa cells to the pro-death activity of simvastatin [172][200].
2.2. Mitochondrial Dynamics
It is now well established that alterations of the mitochondrial structural dynamics (biogenesis, fusion, fission and mitophagy) are deeply involved in the different steps of cancer growth, progression and development of drug resistance [16][60][62][173][174][16,80,82,201,202]. However, current data on the role of the mitochondrial structural alterations in PCa are still scanty.
PGC1α is the well-recognized master regulator of mitochondrial biogenesis [175][203]; it is also involved in the control of the mitochondrial fusion/fission balance by promoting fusion, through the activation of MFN1 and 2, and impairing DRP1 expression, through the binding to its promoter region [176][204]. The expression of this gene, together with the mitochondrial number, was found to be upregulated in tumors, including PCa, of African American cancer patients known to be exposed to a higher risk of cancer and mortality compared to European American patients [177][205]. PGC1α has been observed to be highly expressed in PCa cells harboring either deletion or mutation of the classic tumor suppressor protein p53, and its expression positively correlates with cancer cell metastatic behavior [178][206]. It has been demonstrated that, in CRPC PC3 cells, overexpression of p53 decreases the expression and activity (i.e., nuclear localization) of PGC-1α, thereby leading to a reduced mitochondrial mass and a significant change in the expression levels of genes and proteins involved in the fusion/fission balance [179][207]. More recently, Galbraith and coworkers reported that, in PCa cells, the activity of PGC1α is also regulated by the PPARG/AKT3 axis. Specifically, these authors found that, in CRPC cells, overexpression of the transcription factor PPARG induces the expression of the AKT3 kinase that, in turn, triggers the nuclear localization of PGC1α, thereby driving mitochondrial biogenesis and ATP production which may fuel the metastatic behavior of tumor cells [140][166].
Alterations of the fusion/fission balance have also shown to be deeply involved in tumorigenesis, although the data so far available on this issue in PCa cells are still limited. Generally, it is accepted that mitochondria fission, the division of mitochondria in smaller organelles, is a typical feature of cells undergoing apoptosis; moreover, this process foresees the translocation of the cytoplasmic DRP1 protein to the mitochondria where it interacts with its receptor FIS1. In PCa cells, it has been reported that the overload of Ca2+ in mitochondria triggers the interaction of DRP1 with FIS1, thereby leading to mitochondrial fragmentation and enhanced cell response to pro-apoptotic agents [180][209]. In line with these observations, rwesearchers observed that, in CRPC cells, mitochondrial Ca2+ and ROS overload triggers mitochondrial fission and mitophagy to mediate the pro-death (apoptotic, paraptotic) activities of natural anticancer compounds [58][78]. Moreover, enhanced mitochondrial fusion, together with mutations of the complex I mtDNA, were found to be associated with PCa progression, as evaluated in cancer cell lines as well as in mice and human tissue samples [181][210].
On the other hand, mitochondrial fission has been recently reported to play a key role in the maintenance of stemness features in prostate CSCs. Specifically, Civenni and coworkers focused their attention on bromodomain and extra-terminal domain (BET) proteins, such as bromodomain containing 4 (BRD4), well known as epigenetic modifiers of gene transcription. These authors showed that the DRP1 receptor, and fission factor, MFF is upregulated in hormone-refractory human prostate tumors as well as in prostate CSCs. Moreover, they could show that BRD4 acts as a key driver of MFF transcription and, therefore, of mitochondrial fission, which is an essential biological event for the survival and self-renewal of CSCs; accordingly, they observed that the inhibition of the BRD4 activity and of the subsequent MFF transcription results in the accumulation of dysfunctional mitochondria and, consequently, in the acquisition of the senescent phenotype in these cells. Thus, mitochondrial fission is a crucial process for the maintenance of the self-renewal and tumorigenic potential of the CSC subpopulation in prostate tumors.
