Bone regeneration is a complex process that includes skeletal cells such as osteoblasts, osteoclasts, and immune cells to regulate bone formation and resorption. Osteoimmunology, studying this complicated process, has recently been used to develop biomaterials for advanced bone regeneration. Ideally, a biomaterial shall enable a timely switch from early stage inflammatory (to recruit osteogenic progenitor cells) to later-stage anti-inflammatory (to promote differentiation and terminal osteogenic mineralization and model the microstructure of bone tissue) in immune cells, especially the M1-to-M2 phenotype switch in macrophage populations, for bone regeneration. Nanoparticle (NP)-based advanced drug delivery systems can enable the controlled release of therapeutic reagents and the delivery of therapeutics into specific cell types, thereby benefiting bone regeneration through osteoimmunomodulation.
- nanoparticles
- bone regeneration
- osteoimmunomodulation
1. Application of Nanoparticles in Bone Regeneration
2. Applications of NPs in Osteoimmunomodulation
| In Vitro and In Vivo Assays | Physical and Chemical Characterization | Biocompatibility Evaluation and Biomechanical Analysis |
|---|---|---|
| Cell culture-based assays (osteoblast, osteoclast, macrophage) Enzyme-linked immunosorbent assays (ELISA) Alkaline phosphatase activity assays Mineralization assays |
X-ray diffraction (XRD) Transmission electron microscopy (TEM) Scanning electron microscopy (SEM) Dynamic light scattering (DLS) |
Cytotoxicity assays (MTT, LDH) Hemocompatibility assays (hemolysis, platelet activation) Inflammation assays (IL-1β, TNF-α, IL-6) |
| Implantation studies in animal models (rats, mice, rabbits) Histological analysis (bone formation and resorption) Micro-computed tomography (μCT) Bone density measurements |
Fourier transform infrared spectroscopy (FTIR) Energy-dispersive X-ray spectroscopy (EDS) X-ray fluorescence (XRF) Nuclear magnetic resonance spectroscopy (NMR) |
Contact angle measurements Zeta potential measurements Surface roughness analysis Compression tests Tensile tests Indentation tests |
| Strategies for Regulating Macrophage Polarization | Applications of NPs in Osteoimunomodulation | References |
|---|---|---|
| Intrinsic properties | Gold, TiO2, and cerium oxide (CeO2) NPs can enhance M2 polarization. | [55][56][57] |
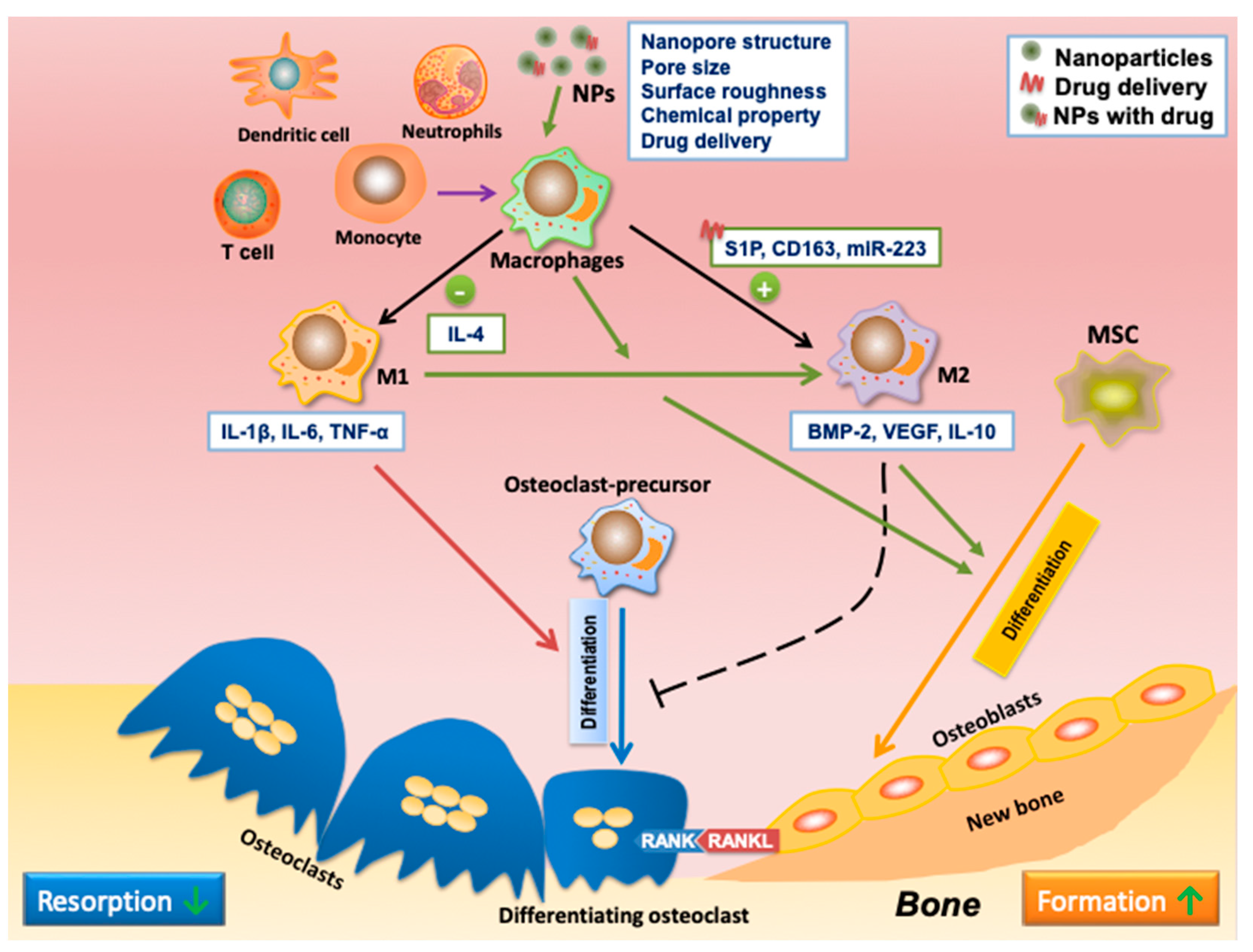
| Nanopore structure and pore size | NPs with pores of larger size (100 and 200 nm) were highly anti-inflammatory and inhibited M1 polarization. | [58] |
| The nanoneedle structure induced M2 polarization. The micropattern sizes of 12 μm and 36 μm in the micro/nano hierarchy enhanced M2 polarization. |
[59] | |
| Surface roughness | Ti with smooth surface stimulated M1 activation. Ti with rough surface enhanced M2 polarization. |
[61] |
| Composition | Gold NPs fused hexapeptides Cys-Leu-Pro-Phe-Phe-Asp, peptide arginine-glycine-aspartic acid (RGD), and IL-4 could stimulate M2 polarization. | [51][65][66] |
| CeO2 NPs with hydroxyapatite could enhance M2 polarization. | [67] | |
| Strontium (Sr)- or copper (Cu)-doped bioactive glass particles promoted M2 polarization and enhanced osteogenesis. | [63][64] | |
| Drug delivery | Various nanocarriers have delivered IL-4 (anti-inflammatory cytokine) to induce M2 polarization. | [70][71][72] |
| NPs can deliver S1P synthetic analog to direct macrophage polarization toward M2. | [73] | |
| CD163 gene has been encapsulated into polyethyleneimine NPs decorated with a mannose ligand to induce CD163 expression and macrophage polarization toward M2. | [74] | |
| miR-223 5p mimic was delivered to induce macrophage polarization to M2. | [75] | |
| Resolvin D1-loaded gold nanocages (AuNC) were coated with M1-like macrophage membranes to enhance M2 polarization. | [76] | |
| A sequential release of therapeutics induces the M1-to-M2 phenotype switch during tissue regeneration. | Spillar et al. designed a scaffold that achieved a sequential release of first IFN-γ and then IL-4 to modulate macrophage polarization from early stage M1 to later-stage M2. | [77] |
| NPs carry both miRNA-155 and miRNA-21 to sequentially stimulate macrophage polarization first toward M1 and then the M2 phenotype. | [78] | |
| Microcrystalline bioactive glass scaffolds with different doses of ZnO orchestrate the sequential M1-to-M2 macrophage polarization. | [79] | |
| Sr-substituted nanohydroxyapatite (nano-SrHA) coatings and IFN-γ to the surface of native SIS membrane control a sequential M1-M2 macrophage transition. | [80] |
References
- Gong, T.; Xie, J.; Liao, J.; Zhang, T.; Lin, S.; Lin, Y. Nanomaterials and Bone Regeneration. Bone Res. 2015, 3, 15029.
- Dyondi, D.; Webster, T.J.; Banerjee, R. A Nanoparticulate Injectable Hydrogel as a Tissue Engineering Scaffold for Multiple Growth Factor Delivery for Bone Regeneration. Int. J. Nanomed. 2013, 8, 47–59.
- Xu, H.H.K.; Weir, M.D.; Simon, C.G. Injectable and Strong Nano-Apatite Scaffolds for Cell/Growth Factor Delivery and Bone Regeneration. Dent. Mater. 2008, 24, 1212–1222.
- Cai, L.; Guinn, A.S.; Wang, S. Exposed Hydroxyapatite Particles on the Surface of Photo-Crosslinked Nanocomposites for Promoting MC3T3 Cell Proliferation and Differentiation. Acta Biomater. 2011, 7, 2185–2199.
- Zhang, T.; Gao, Y.; Cui, W.; Li, Y.; Xiao, D.; Zhou, R. Nanomaterials-Based Cell Osteogenic Differentiation and Bone Regeneration. Curr. Stem Cell Res. Ther. 2021, 16, 36–47.
- Wu, Y.; Jiang, W.; Wen, X.; He, B.; Zeng, X.; Wang, G.; Gu, Z. A Novel Calcium Phosphate Ceramic–Magnetic Nanoparticle Composite as a Potential Bone Substitute. Biomed. Mater. 2010, 5, 015001.
- Lv, L.; Liu, Y.; Zhang, P.; Zhang, X.; Liu, J.; Chen, T.; Su, P.; Li, H.; Zhou, Y. The Nanoscale Geometry of TiO2 Nanotubes Influences the Osteogenic Differentiation of Human Adipose-Derived Stem Cells by Modulating H3K4 Trimethylation. Biomaterials 2015, 39, 193–205.
- Gaharwar, A.K.; Mihaila, S.M.; Swami, A.; Patel, A.; Sant, S.; Reis, R.L.; Marques, A.P.; Gomes, M.E.; Khademhosseini, A. Bioactive Silicate Nanoplatelets for Osteogenic Differentiation of Human Mesenchymal Stem Cells. Adv. Mater. 2013, 25, 3329–3336.
- Kim, B.-S.; Yang, S.-S.; Kim, C.S. Incorporation of BMP-2 Nanoparticles on the Surface of a 3D-Printed Hydroxyapatite Scaffold Using an ε-Polycaprolactone Polymer Emulsion Coating Method for Bone Tissue Engineering. Colloids Surf. B Biointerfaces 2018, 170, 421–429.
- Wang, L.; Xu, W.; Chen, Y.; Wang, J. Alveolar Bone Repair of Rhesus Monkeys by Using BMP-2 Gene and Mesenchymal Stem Cells Loaded Three-Dimensional Printed Bioglass Scaffold. Sci. Rep. 2019, 9, 18175.
- Poth, N.; Seiffart, V.; Gross, G.; Menzel, H.; Dempwolf, W. Biodegradable Chitosan Nanoparticle Coatings on Titanium for the Delivery of BMP-2. Biomolecules 2015, 5, 3–19.
- Moradikhah, F.; Doosti-Telgerd, M.; Shabani, I.; Soheili, S.; Dolatyar, B.; Seyedjafari, E. Microfluidic Fabrication of Alendronate-Loaded Chitosan Nanoparticles for Enhanced Osteogenic Differentiation of Stem Cells. Life Sci. 2020, 254, 117768.
- Liu, J.; Cui, Y.; Kuang, Y.; Xu, S.; Lu, Q.; Diao, J.; Zhao, N. Hierarchically Porous Calcium–Silicon Nanosphere-Enabled Co-Delivery of MicroRNA-210 and Simvastatin for Bone Regeneration. J. Mater. Chem. B 2021, 9, 3573–3583.
- Seddighian, A.; Ganji, F.; Baghaban-Eslaminejad, M.; Bagheri, F. Electrospun PCL Scaffold Modified with Chitosan Nanoparticles for Enhanced Bone Regeneration. Prog. Biomater. 2021, 10, 65–76.
- Balagangadharan, K.; Chandran, S.V.; Arumugam, B.; Saravanan, S.; Venkatasubbu, G.D.; Selvamurugan, N. Chitosan/Nano-Hydroxyapatite/Nano-Zirconium Dioxide Scaffolds with MiR-590-5p for Bone Regeneration. Int. J. Biol. Macromol. 2018, 111, 953–958.
- Bu, W.; Xu, X.; Wang, Z.; Jin, N.; Liu, L.; Liu, J.; Zhu, S.; Zhang, K.; Jelinek, R.; Zhou, D. Ascorbic Acid-PEI Carbon Dots with Osteogenic Effects as MiR-2861 Carriers to Effectively Enhance Bone Regeneration. ACS Appl. Mater. Interfaces 2020, 12, 50287–50302.
- Thamake, S.I.; Raut, S.L.; Gryczynski, Z.; Ranjan, A.P.; Vishwanatha, J.K. Alendronate Coated Poly-Lactic-Co-Glycolic Acid (PLGA) Nanoparticles for Active Targeting of Metastatic Breast Cancer. Biomaterials 2012, 33, 7164–7173.
- Cenni, E.; Granchi, D.; Avnet, S.; Fotia, C.; Salerno, M.; Micieli, D.; Sarpietro, M.G.; Pignatello, R.; Castelli, F.; Baldini, N. Biocompatibility of Poly(d,l-Lactide-Co-Glycolide) Nanoparticles Conjugated with Alendronate. Biomaterials 2008, 29, 1400–1411.
- Hwang, S.-J.; Lee, J.-S.; Ryu, T.-K.; Kang, R.-H.; Jeong, K.-Y.; Jun, D.-R.; Koh, J.-M.; Kim, S.-E.; Choi, S.-W. Alendronate-Modified Hydroxyapatite Nanoparticles for Bone-Specific Dual Delivery of Drug and Bone Mineral. Macromol. Res. 2016, 24, 623–628.
- Wang, B.; Guo, Y.; Chen, X.; Zeng, C.; Hu, Q.; Yin, W.; Li, W.; Xie, H.; Zhang, B.; Huang, X.; et al. Nanoparticle-Modified Chitosan-Agarose-Gelatin Scaffold for Sustained Release of SDF-1 and BMP-2. Int. J. Nanomed. 2018, 13, 7395–7408.
- Heparin-Regulated Release of Growth Factors in Vitro and Angiogenic Response in Vivo to Implanted Hyaluronan Hydrogels Containing VEGF and BFGF-ScienceDirect. Available online: https://www.sciencedirect.com/science/article/abs/pii/S014296120600456X (accessed on 24 September 2022).
- Kang, W.; Yun, Y.-R.; Lee, D.-S.; Kim, T.-H.; Kim, J.-H.; Kim, H.-W.; Jang, J.-H. Fluorescence-Based Retention Assays Reveals Sustained Release of Vascular Endothelial Growth Factor from Bone Grafts. J. Biomed. Mater. Res. Part A 2016, 104, 283–290.
- Geuze, R.E.; Theyse, L.F.; Kempen, D.H.; Hazewinkel, H.A.; Kraak, H.Y.; Öner, F.C.; Dhert, W.J.; Alblas, J. A Differential Effect of Bone Morphogenetic Protein-2 and Vascular Endothelial Growth Factor Release Timing on Osteogenesis at Ectopic and Orthotopic Sites in a Large-Animal Model. Tissue Eng. Part A 2012, 18, 2052–2062.
- Cao, L.; Kong, X.; Lin, S.; Zhang, S.; Wang, J.; Liu, C.; Jiang, X. Synergistic Effects of Dual Growth Factor Delivery from Composite Hydrogels Incorporating 2-N, 6-O-Sulphated Chitosan on Bone Regeneration. Artif. Cells Nanomed. Biotechnol. 2018, 46, S1–S17.
- Talavera-Adame, D.; Wu, G.; He, Y.; Ng, T.T.; Gupta, A.; Kurtovic, S.; Hwang, J.Y.; Farkas, D.L.; Dafoe, D.C. Endothelial Cells in Co-Culture Enhance Embryonic Stem Cell Differentiation to Pancreatic Progenitors and Insulin-Producing Cells through BMP Signaling. Stem Cell Rev. Rep. 2011, 7, 532–543.
- Young Park, J.; Shim, J.-H.; Choi, S.-A.; Jang, J.; Kim, M.; Hwa Lee, S.; Cho, D.-W. 3D Printing Technology to Control BMP-2 and VEGF Delivery Spatially and Temporally to Promote Large-Volume Bone Regeneration. J. Mater. Chem. B 2015, 3, 5415–5425.
- Kempen, D.H.; Lu, L.; Heijink, A.; Hefferan, T.E.; Creemers, L.B.; Maran, A.; Yaszemski, M.J.; Dhert, W.J. Effect of Local Sequential VEGF and BMP-2 Delivery on Ectopic and Orthotopic Bone Regeneration. Biomaterials 2009, 30, 2816–2825.
- Dou, D.D.; Zhou, G.; Liu, H.W.; Zhang, J.; Liu, M.L.; Xiao, X.F.; Fei, J.J.; Guan, X.L.; Fan, Y.B. Sequential Releasing of VEGF and BMP-2 in Hydroxyapatite Collagen Scaffolds for Bone Tissue Engineering: Design and Characterization. Int. J. Biol. Macromol. 2019, 123, 622–628.
- Kupikowska-Stobba, B.; Kasprzak, M. Fabrication of Nanoparticles for Bone Regeneration: New Insight into Applications of Nanoemulsion Technology. J. Mater. Chem. B 2021, 9, 5221–5244.
- Nirmala, R.; Sheikh, F.A.; Kanjwal, M.A.; Lee, J.H.; Park, S.-J.; Navamathavan, R.; Kim, H.Y. Synthesis and Characterization of Bovine Femur Bone Hydroxyapatite Containing Silver Nanoparticles for the Biomedical Applications. J. Nanopart. Res. 2011, 13, 1917–1927.
- Baba Ismail, Y.M.; Ferreira, A.M.; Bretcanu, O.; Dalgarno, K.; El Haj, A.J. Polyelectrolyte Multi-Layers Assembly of SiCHA Nanopowders and Collagen Type I on Aminolysed PLA Films to Enhance Cell-Material Interactions. Colloids Surf. B Biointerfaces 2017, 159, 445–453.
- Narciso, A.M.; da Rosa, C.G.; Nunes, M.R.; Sganzerla, W.G.; Hansen, C.M.; de Melo, A.P.Z.; Paes, J.V.; Bertoldi, F.C.; Barreto, P.L.M.; Masiero, A.V. Antimicrobial Green Silver Nanoparticles in Bone Grafts Functionalization for Biomedical Applications. Biocatal. Agric. Biotechnol. 2021, 35, 102074.
- Cheng, H.; Chawla, A.; Yang, Y.; Li, Y.; Zhang, J.; Jang, H.L.; Khademhosseini, A. Development of Nanomaterials for Bone-Targeted Drug Delivery. Drug Discov. Today 2017, 22, 1336–1350.
- Zhou, X.; Cornel, E.J.; He, S.; Du, J. Recent Advances in Bone-Targeting Nanoparticles for Biomedical Applications. Mater. Chem. Front. 2021, 5, 6735–6759.
- Ossipov, D.A. Bisphosphonate-Modified Biomaterials for Drug Delivery and Bone Tissue Engineering. Expert Opin. Drug Deliv. 2015, 12, 1443–1458.
- Leu, C.-T.; Luegmayr, E.; Freedman, L.P.; Rodan, G.A.; Reszka, A.A. Relative Binding Affinities of Bisphosphonates for Human Bone and Relationship to Antiresorptive Efficacy. Bone 2006, 38, 628–636.
- Zhang, J.; Liu, X.; Deng, T.; Yao, P.; Song, H.; Zhou, S.; Yan, W. Development of Drug Loaded Nanoparticles Binding to Hydroxyapatite Based on a Bisphosphonate Modified Nonionic Surfactant. J. Nanomater. 2015, 16, 145. Available online: https://dl.acm.org/doi/abs/10.1155/2015/393968 (accessed on 24 September 2022).
- de Miguel, L.; Noiray, M.; Surpateanu, G.; Iorga, B.I.; Ponchel, G. Poly(γ-Benzyl-l-Glutamate)-PEG-Alendronate Multivalent Nanoparticles for Bone Targeting. Int. J. Pharm. 2014, 460, 73–82.
- Lee, M.-S.; Su, C.-M.; Yeh, J.-C.; Wu, P.-R.; Tsai, T.-Y.; Lou, S.-L. Synthesis of Composite Magnetic Nanoparticles Fe3O4 with Alendronate for Osteoporosis Treatment. Int. J. Nanomed. 2016, 11, 4583–4594.
- Piao, H.; Kim, M.H.; Cui, M.; Choi, G.; Choy, J.-H. Alendronate-Anionic Clay Nanohybrid for Enhanced Osteogenic Proliferation and Differentiation. J. Korean Med. Sci. 2019, 34, e37.
- Sadeghi, L.; Tanwir, F.; Yousefi Babadi, V. Antioxidant Effects of Alfalfa Can Improve Iron Oxide Nanoparticle Damage: Invivo and Invitro Studies. Regul. Toxicol. Pharmacol. 2016, 81, 39–46.
- Malachowski, T.; Hassel, A. Engineering Nanoparticles to Overcome Immunological Barriers for Enhanced Drug Delivery. Eng. Regen. 2020, 1, 35–50.
- Park, M.V.D.Z.; Neigh, A.M.; Vermeulen, J.P.; de la Fonteyne, L.J.J.; Verharen, H.W.; Briedé, J.J.; van Loveren, H.; de Jong, W.H. The Effect of Particle Size on the Cytotoxicity, Inflammation, Developmental Toxicity and Genotoxicity of Silver Nanoparticles. Biomaterials 2011, 32, 9810–9817.
- Cao, H. Silver Nanoparticles for Antibacterial Devices: Biocompatibility and Toxicity; Taylor & Francis Group: London, UK, 2017; ISBN 978-1-315-35347-0.
- Chen, Z.; Klein, T.; Murray, R.Z.; Crawford, R.; Chang, J.; Wu, C.; Xiao, Y. Osteoimmunomodulation for the Development of Advanced Bone Biomaterials. Mater. Today 2016, 19, 304–321.
- Xie, Y.; Hu, C.; Feng, Y.; Li, D.; Ai, T.; Huang, Y.; Chen, X.; Huang, L.; Tan, J. Osteoimmunomodulatory Effects of Biomaterial Modification Strategies on Macrophage Polarization and Bone Regeneration. Regen. Biomater. 2020, 7, 233–245.
- Song, G.; SPetschauer, J.; JMadden, A.; CZamboni, W. Nanoparticles and the Mononuclear Phagocyte System: Pharmacokinetics and Applications for Inflammatory Diseases. Curr. Rheumatol. Rev. 2014, 10, 22–34.
- Liu, Y.; Hardie, J.; Zhang, X.; Rotello, V.M. Effects of Engineered Nanoparticles on the Innate Immune System. Semin. Immunol. 2017, 34, 25–32.
- Zhao, Y.-J.; Gao, Z.-C.; He, X.-J.; Li, J. The Let-7f-5p–Nme4 Pathway Mediates Tumor Necrosis Factor α-Induced Impairment in Osteogenesis of Bone Marrow-Derived Mesenchymal Stem Cells. Biochem. Cell Biol. 2021, 99, 488–498.
- Ono, T.; Takayanagi, H. Osteoimmunology in Bone Fracture Healing. Curr. Osteoporos. Rep. 2017, 15, 367–375.
- Wang, L.; Zhang, H.; Sun, L.; Gao, W.; Xiong, Y.; Ma, A.; Liu, X.; Shen, L.; Li, Q.; Yang, H. Manipulation of Macrophage Polarization by Peptide-Coated Gold Nanoparticles and Its Protective Effects on Acute Lung Injury. J. Nanobiotechnol. 2020, 18, 38.
- Rifas, L. T-Cell Cytokine Induction of BMP-2 Regulates Human Mesenchymal Stromal Cell Differentiation and Mineralization. J. Cell. Biochem. 2006, 98, 706–714.
- Freytes, D.O.; Wan, L.Q.; Vunjak-Novakovic, G. Geometry and Force Control of Cell Function. J. Cell. Biochem. 2009, 108, 1047–1058.
- Schett, G. Osteoimmunology in Rheumatic Diseases. Arthritis Res. Ther. 2009, 11, 210.
- Shen, X.; Yu, Y.; Ma, P.; Luo, Z.; Hu, Y.; Li, M.; He, Y.; Zhang, Y.; Peng, Z.; Song, G. Titania Nanotubes Promote Osteogenesis via Mediating Crosstalk between Macrophages and MSCs under Oxidative Stress. Colloids Surf. B Biointerfaces 2019, 180, 39–48.
- Lee, C.-H.; Kim, Y.-J.; Jang, J.-H.; Park, J.-W. Modulating Macrophage Polarization with Divalent Cations in Nanostructured Titanium Implant Surfaces. Nanotechnology 2016, 27, 085101.
- Chigurupati, S.; Mughal, M.R.; Okun, E.; Das, S.; Kumar, A.; McCaffery, M.; Seal, S.; Mattson, M.P. Effects of Cerium Oxide Nanoparticles on the Growth of Keratinocytes, Fibroblasts and Vascular Endothelial Cells in Cutaneous Wound Healing. Biomaterials 2013, 34, 2194–2201.
- Chen, Z.; Ni, S.; Han, S.; Crawford, R.; Lu, S.; Wei, F.; Chang, J.; Wu, C.; Xiao, Y. Nanoporous Microstructures Mediate Osteogenesis by Modulating the Osteo-Immune Response of Macrophages. Nanoscale 2017, 9, 706–718.
- Yang, C.; Zhao, C.; Wang, X.; Shi, M.; Zhu, Y.; Jing, L.; Wu, C.; Chang, J. Stimulation of Osteogenesis and Angiogenesis by Micro/Nano Hierarchical Hydroxyapatite via Macrophage Immunomodulation. Nanoscale 2019, 11, 17699–17708.
- Almatroudi, A. Silver Nanoparticles: Synthesis, Characterisation and Biomedical Applications. Open Life Sci. 2020, 15, 819–839.
- Hotchkiss, K.M.; Reddy, G.B.; Hyzy, S.L.; Schwartz, Z.; Boyan, B.D.; Olivares-Navarrete, R. Titanium Surface Characteristics, Including Topography and Wettability, Alter Macrophage Activation. Acta Biomater. 2016, 31, 425–434.
- Cai, D.; Gao, W.; Li, Z.; Zhang, Y.; Xiao, L.; Xiao, Y. Current Development of Nano-Drug Delivery to Target Macrophages. Biomedicines 2022, 10, 1203.
- Zhang, W.; Zhao, F.; Huang, D.; Fu, X.; Li, X.; Chen, X. Strontium-Substituted Submicrometer Bioactive Glasses Modulate Macrophage Responses for Improved Bone Regeneration. ACS Appl. Mater. Interfaces 2016, 8, 30747–30758.
- Lin, R.; Deng, C.; Li, X.; Liu, Y.; Zhang, M.; Qin, C.; Yao, Q.; Wang, L.; Wu, C. Copper-Incorporated Bioactive Glass-Ceramics Inducing Anti-Inflammatory Phenotype and Regeneration of Cartilage/Bone Interface. Theranostics 2019, 9, 6300–6313.
- Bartneck, M.; Ritz, T.; Keul, H.A.; Wambach, M.; Bornemann, J.; Gbureck, U.; Ehling, J.; Lammers, T.; Heymann, F.; Gassler, N. Peptide-Functionalized Gold Nanorods Increase Liver Injury in Hepatitis. Acs Nano 2012, 6, 8767–8777.
- Raimondo, T.M.; Mooney, D.J. Functional Muscle Recovery with Nanoparticle-Directed M2 Macrophage Polarization in Mice. Proc. Natl. Acad. Sci. USA 2018, 115, 10648–10653.
- Li, K.; Shen, Q.; Xie, Y.; You, M.; Huang, L.; Zheng, X. Incorporation of Cerium Oxide into Hydroxyapatite Coating Regulates Osteogenic Activity of Mesenchymal Stem Cell and Macrophage Polarization. J. Biomater. Appl. 2017, 31, 1062–1076.
- Nanostructured Surface Modification to Bone Implants for Bone Reg...: Ingenta Connect. Available online: https://www.ingentaconnect.com/contentone/asp/jbn/2018/00000014/00000004/art00002 (accessed on 1 February 2023).
- Zhang, W.; Huang, D.; Zhao, F.; Gao, W.; Sun, L.; Li, X.; Chen, X. Synergistic Effect of Strontium and Silicon in Strontium-Substituted Sub-Micron Bioactive Glass for Enhanced Osteogenesis. Mater. Sci. Eng. C 2018, 89, 245–255.
- IL-4 Administration Exerts Preventive Effects via Suppression of Underlying Inflammation and TNF-α-Induced Apoptosis in Steroid-Induced Osteonecrosis; SpringerLink: Berlin, Germany, 2016; Available online: https://link.springer.com/article/10.1007/s00198-015-3474-6 (accessed on 24 September 2022).
- He, X.-T.; Li, X.; Xia, Y.; Yin, Y.; Wu, R.-X.; Sun, H.-H.; Chen, F.-M. Building Capacity for Macrophage Modulation and Stem Cell Recruitment in High-Stiffness Hydrogels for Complex Periodontal Regeneration: Experimental Studies in Vitro and in Rats. Acta Biomater. 2019, 88, 162–180.
- Kwon, D.; Cha, B.G.; Cho, Y.; Min, J.; Park, E.-B.; Kang, S.-J.; Kim, J. Extra-Large Pore Mesoporous Silica Nanoparticles for Directing in Vivo M2 Macrophage Polarization by Delivering IL-4. Nano Lett. 2017, 17, 2747–2756.
- Hughes, J.E.; Srinivasan, S.R.; Hedrick, C.C. Sphingosine-1-Phosphate Induces an Anti-Inflammatory Phenotype in Macrophages During Inflammation. FASEB J. 2017, 21, A772. Available online: https://faseb.onlinelibrary.wiley.com/doi/abs/10.1096/fasebj.21.6.A772-b (accessed on 24 September 2022).
- Alvarado-Vazquez, P.A.; Bernal, L.; Paige, C.A.; Grosick, R.L.; Vilrriales, C.M.; Ferreira, D.W.; Ulecia-Morón, C.; Romero-Sandoval, E.A. Macrophage-Specific Nanotechnology-Driven CD163 Overexpression in Human Macrophages Results in an M2 Phenotype under Inflammatory Conditions. Immunobiology 2017, 222, 900–912.
- Saleh, B.; Dhaliwal, H.K.; Portillo-Lara, R.; Shirzaei Sani, E.; Abdi, R.; Amiji, M.M.; Annabi, N. Local Immunomodulation Using an Adhesive Hydrogel Loaded with MiRNA-Laden Nanoparticles Promotes Wound Healing. Small 2019, 15, 1902232.
- Yin, C.; Zhao, Q.; Li, W.; Zhao, Z.; Wang, J.; Deng, T.; Zhang, P.; Shen, K.; Li, Z.; Zhang, Y. Biomimetic Anti-Inflammatory Nano-Capsule Serves as a Cytokine Blocker and M2 Polarization Inducer for Bone Tissue Repair. Acta Biomater. 2020, 102, 416–426.
- Spiller, K.L.; Nassiri, S.; Witherel, C.E.; Anfang, R.R.; Ng, J.; Nakazawa, K.R.; Yu, T.; Vunjak-Novakovic, G. Sequential Delivery of Immunomodulatory Cytokines to Facilitate the M1-to-M2 Transition of Macrophages and Enhance Vascularization of Bone Scaffolds. Biomaterials 2015, 37, 194–207.
- Li, X.; Xue, S.; Zhan, Q.; Sun, X.; Chen, N.; Li, S.; Zhao, J.; Hou, X.; Yuan, X. Sequential Delivery of Different MicroRNA Nanocarriers Facilitates the M1-to-M2 Transition of Macrophages. ACS Omega 2022, 7, 8174–8183.
- Bai, X.; Liu, W.; Xu, L.; Ye, Q.; Zhou, H.; Berg, C.; Yuan, H.; Li, J.; Xia, W. Sequential Macrophage Transition Facilitates Endogenous Bone Regeneration Induced by Zn-Doped Porous Microcrystalline Bioactive Glass. J. Mater. Chem. B 2021, 9, 2885–2898.
- Yang, L.; Zhou, J.; Yu, K.; Yang, S.; Sun, T.; Ji, Y.; Xiong, Z.; Guo, X. Surface Modified Small Intestinal Submucosa Membrane Manipulates Sequential Immunomodulation Coupled with Enhanced Angio-and Osteogenesis towards Ameliorative Guided Bone Regeneration. Mater. Sci. Eng. C 2021, 119, 111641.
- Das, A.; Segar, C.E.; Hughley, B.B.; Bowers, D.T.; Botchwey, E.A. The Promotion of Mandibular Defect Healing by the Targeting of S1P Receptors and the Recruitment of Alternatively Activated Macrophages. Biomaterials 2013, 34, 9853–9862.
- Etzerodt, A.; Moestrup, S.K. CD163 and Inflammation: Biological, Diagnostic, and Therapeutic Aspects. Antioxid. Redox Signal. 2013, 18, 2352–2363.
- Wang, S.; Xiao, L.; Prasadam, I.; Crawford, R.; Zhou, Y.; Xiao, Y. Inflammatory Macrophages Interrupt Osteocyte Maturation and Mineralization via Regulating the Notch Signaling Pathway. Mol. Med. 2022, 28, 102.
- Qiao, W.; Xie, H.; Fang, J.; Shen, J.; Li, W.; Shen, D.; Wu, J.; Wu, S.; Liu, X.; Zheng, Y. Sequential Activation of Heterogeneous Macrophage Phenotypes Is Essential for Biomaterials-Induced Bone Regeneration. Biomaterials 2021, 276, 121038.
- Cai, X.; Yin, Y.; Li, N.; Zhu, D.; Zhang, J.; Zhang, C.-Y.; Zen, K. Re-Polarization of Tumor-Associated Macrophages to pro-Inflammatory M1 Macrophages by MicroRNA-155. J. Mol. Cell Biol. 2012, 4, 341–343.
- Jablonski, K.A.; Gaudet, A.D.; Amici, S.A.; Popovich, P.G.; Guerau-de-Arellano, M. Control of the Inflammatory Macrophage Transcriptional Signature by MiR-155. PloS ONE 2016, 11, e0159724.
- MicroRNA-155 Inhibits Polarization of Macrophages to M2-Type and Suppresses Choroidal Neovascularization; SpringerLink: Berlin, Germany, 2018; Available online: https://link.springer.com/article/10.1007/s10753-017-0672-8 (accessed on 25 September 2022).
- Gao, H.; Dai, W.; Zhao, L.; Min, J.; Wang, F. The Role of Zinc and Zinc Homeostasis in Macrophage Function. J. Immunol. Res. 2018, 2018, e6872621.
- Liu, M.-J.; Bao, S.; Gálvez-Peralta, M.; Pyle, C.J.; Rudawsky, A.C.; Pavlovicz, R.E.; Killilea, D.W.; Li, C.; Nebert, D.W.; Wewers, M.D.; et al. ZIP8 Regulates Host Defense through Zinc-Mediated Inhibition of NF-ΚB. Cell Rep. 2013, 3, 386–400.
- Díez-Pascual, A.M. Surface Engineering of Nanomaterials with Polymers, Biomolecules, and Small Ligands for Nanomedicine. Materials 2022, 15, 3251.
