Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Sajid Ali.
Lipases are versatile biocatalysts and are used in different bioconversion reactions. Microbial lipases are attracting a great amount of attention due to the rapid advancement of enzyme technology and its practical application in a variety of industrial processes. Lipase purification is a meticulous technique that requires great care to retain the bioactive state of the lipase.
- microbial lipase
- purification
- lipase engineering
- biocatalysis
1. Introduction
Lipase purification is a meticulous technique that requires great care to retain the bioactive state of the lipase. Lipases have been purified from various microbial origins to a level of homogeneity by using different purification techniques. These purification strategies can be broadly divided into two categories: (A) classical purification techniques and (B) modern purification techniques. The classical techniques are usually non-specific, laborious, and multi-step, and the purity level achieved is not adequate. On the other hand, modern purification techniques are stress-free, specific, large-scale, and can reach high purity levels. Improvements in purification strategies have paved the way for a variety of choices in the design of highly specialized purification techniques for different microbial lipases. The purification of the lipases to a level of homogeneity helped in the identification sequence of the amino acids and their 3D structure, which led to a better understanding of their unique characteristics when used in different reactions.
2. Classical Purification Techniques
Precipitation and Chromatographic Separation
Initially, filtration or centrifugation procedures were employed for the purification of lipases from a culture broth. Microbial lipases are mostly extracellular and fermentation is followed by the separation of the cells from the culture media. During the purification process, precipitation is the initial step which is followed by chromatographic separation techniques. Almost all purification schemes use a precipitation step in which ammonium sulfate, ethanol, acetone, or hydrochloric acid are most commonly used. According to the literature available on the subject, the precipitation step has been attempted in more than 80% of the purification schemes, in which ammonium sulfate is used twice (60%) as often as ethanol or acetone (30%). Precipitation is followed by a combination of various chromatographic techniques such as affinity chromatography and gel filtration [80][1].
A recent study by Bharathi et al. [81][2] revealed the use of a cell-free supernatant as a crude enzyme for the precipitation and dialysis process, wherein ammonium sulfate fractions from 20 to 80% (w/v) were used. It was concluded that an ammonium sulfate fraction from 40–60% (w/v) showed a higher lipase precipitation activity compared to the other fractions used in the study. The precipitation enzymes were then purified using a dialysis membrane. While lipase activity is boosted with the increasing concentrations of the ammonium sulfate solution, the above study also revealed that increasing the saturation of ammonium sulfate above 80% (w/v) halted the activity. More interestingly, precipitation procedures have high average yields (87%) compared to other techniques [80][1]. In addition, halophilic lipase purification using three steps involving precipitation with ammonium sulfate and performing ion-exchange chromatography twice was reported [82][3].
Generally, a single chromatographic procedure is insufficient and various combinations of chromatographic steps are used to achieve the required level of purity. The methods used most frequently are ion exchange, affinity, and adsorption chromatographies [80][1]. The selection of the appropriate chromatographic method depends on the lipase preparation and purification scheme. The ion exchangers routinely used are the diethyl amino ethyl (DEAE) group in anion exchange and the carboxy methyl (CM) group in cation exchange. This is followed by the gel filtration purification method [80,83,84][1][4][5]. Modern purification techniques are employed for lipase purification to improve lipase productivity and reduce processing time. Developments in purification procedures have widened the choices available for selection when designing specialized purification schemes for microbial lipases.
3. Modern Purification Techniques
The advantages of modern purification techniques are primarily due to their high yields and shorter process times as compared to classical purification techniques. Currently, the industrial sector is seeking lipase purification techniques that are economical, speedy, and offer a high yield.
3.1. Immunopurification
The application of affinity chromatography for the purification of a target protein by using an antibody–antigen system is known as immunopurification (Figure 1). This novel technique is also known as immunoaffinity chromatography, which can be applied for the robust separation of specific proteins. In immunopurification, affinity-purified polyclonal and monoclonal antibodies are used for the separation of target proteins.
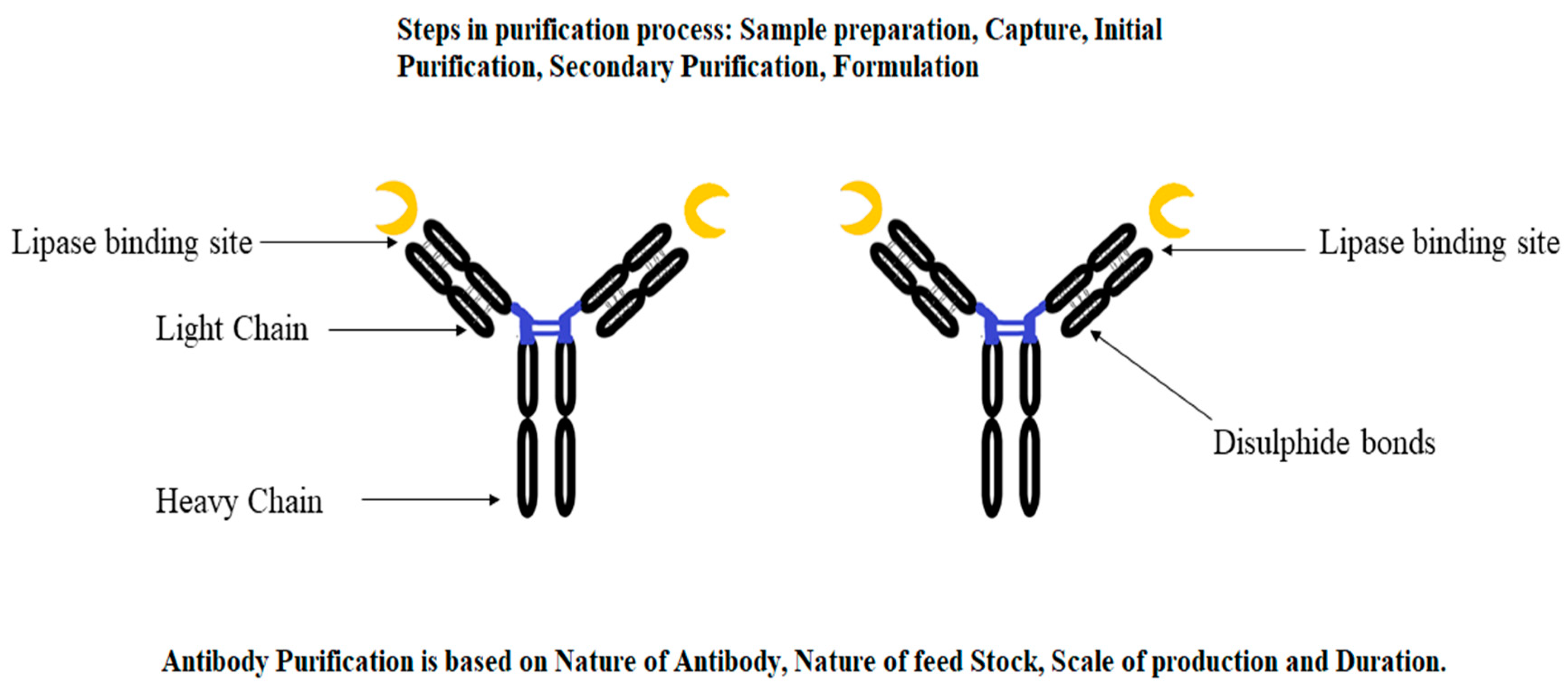
Figure 1.
A representative diagram of an antibody used for the immunopurification of microbial lipases.
Initially, this was regarded as an expensive technique. However, new developments in antibody mass production have paved the way for the deployment of immunopurification at the industrial level by using different monoclonal antibodies [85,86][6][7]. A study by Rahimi et al. [87][8] revealed two monoclonal antibodies (MoAbs), BF11 and VNH9, that immobilize lipase obtained from Candida rugosa, wherein both antibodies of the IgG1 isotype recognize specific antigenic determinants shared by different C. rugosa lipase isoforms. The residual activity of lipase was found to be 99% (for BF11) and 92% (in the case of VNH9) after forming an immune complex [19,87][8][9]. This showed the potential of monoclonal antibodies for application in the purification of microbial lipases.
3.2. Reverse Micellar Systems (RMS)
Reversed micelles are associated with the idea of a microreactor where the enzyme can be protected from the detrimental effects of the solvent. The structure of a reverse micelle consists of an aqueous microdomain facing the polar head of the surfactants that surround this core and interact with the bulk organic solvent, which is non-polar, through hydrophobic chains [21,88][10][11]. The reverse micellar system (RMS) comprises two essential steps known as forward and reverse extraction (Figure 2). In the liquid–liquid separation process of forward extraction, lipase is transported from an aqueous medium to an organic phase where the enzyme is encapsulated in reversed micelles and protected from denaturation upon contact with the organic solvent, while in reverse extraction, the lipase does not remain in the reverse micelles for a long time but is simply transported into an aqueous media without any contaminant. Recently, surfactants are routinely used in RMS because they enhance the solubility of organic compounds and reduce surface tension [89,90][12][13]. In the context of industrial enzyme uses, the question of whether and how lipases and surfactants interact frequently arises. The active conformation of some lipases has been demonstrated to be stabilized by non-ionic surfactants that mimic the lipid substrate, although anionic surfactants’ catalytic interactions have not been thoroughly studied [91][14]. With a hydrophilic head group attached to a hydrophobic tail, surfactant structures resemble the natural substrates of lipases. Due to this resemblance, surfactants are also used to capture the open conformation of the lipases during crystallization studies. For instance, the open form of a member of the thermoalkalophilic lipase family’s structure was recorded when non-ionic surfactants were present. More recently, Shehata et al. [91][14] reported that no experimentally obtained ionic surfactant-captured lipase structures exist, despite the fact that a number of studies have shown that ionic surfactants stabilize lipases [88][11].
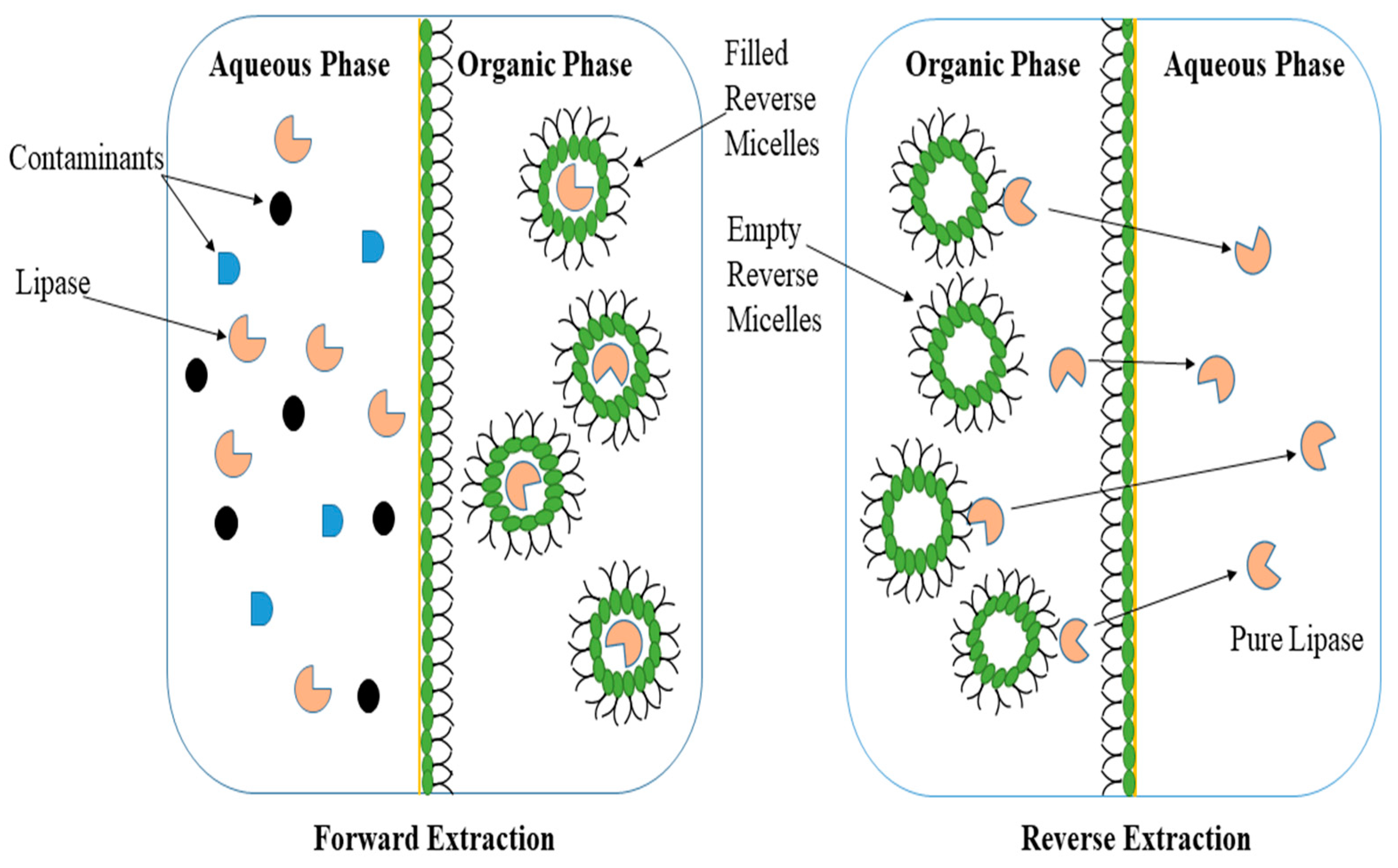
Figure 2.
A schematic diagram of the two-step extraction in/with micellar systems.
In RMS, surfactants expand the interfacial area of the solvent which augments micelle formation and enzyme recovery from the system. This role of the surfactants and the formation of the micelle pave the way for robust downstream processing and a higher degree of enzyme purification [90][13]. Gaikaiwari et al. [89][12] applied both the classical approach and reverse micellar approach for the purification of lipase from Pseudomonas and concluded that the RMS approach resulted in a 15-fold higher purification with 80% recovery in 45 min. Conversely, the conventional system resulted in 52% enzyme recovery and required 30–40 h. The RMS exhibited the fastest and most economical procedure for lipase purification from bacterial sources. Similarly, the role of lipase from a thermophilic fungus, Thermomyces lanuginosus, was reported by Fernandes et al. [92][15] in the hydrolysis and synthesis reaction in an RMS which produced a higher yield (200 U/mg) in a short period of time.
3.3. Aqueous Two-Phase System (ATPS)
The ATPS technique has achieved prominence in purification technology and the application of this technique has resulted in rapid developments in the separation and purification of biomolecules. ATPS is a single-step technique with high selectivity and an easy scale-up process. ATPS is one of the more refined systems for the purification of biomolecules such as DNA, RNA, proteins, and other cellular components. The unique characteristics of this system include a continuous mode of operation, low toxicity of the phase-forming chemicals, and high biocompatibility. The ATPS is a liquid-to-liquid extraction strategy that requires lower energy and provides a relatively quick separation of the target biomolecules into two distinct phases (Figure 3). The purification of the biomolecules is based on the incompatibility of the two phases and the ATPS exploits this unique characteristic for extraction and purification.
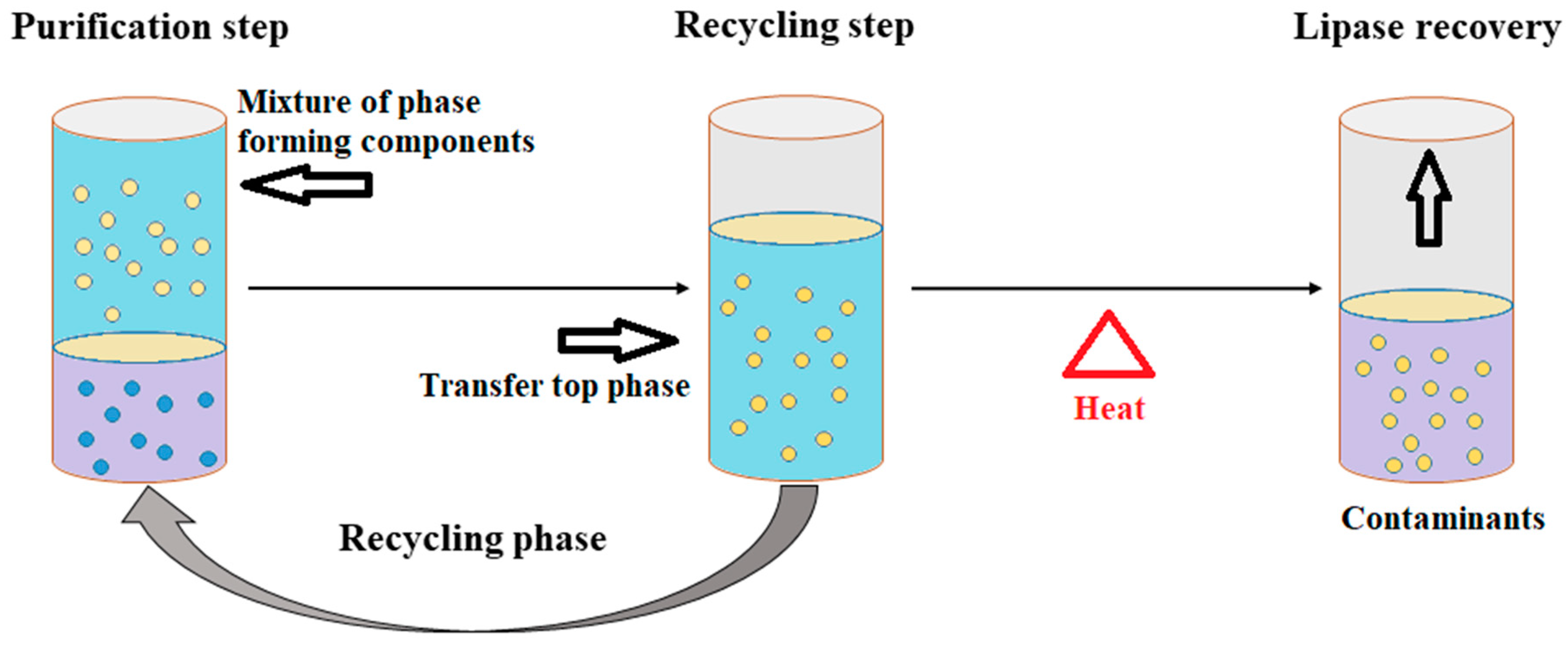
Figure 3.
Schematic presentation of an aqueous two-phase system (ATPS) for lipase purification.
In the ATPS, polymers and salt are used for phase separation. The most common polymer-based ATPSs use dextran and polyethylene glycol (PEG), while polymer–salt ATPSs include the PEG–magnesium sulfate ATPS and PEG–potassium phosphate ATPS [93][16]. Souza et al. [94][17] used an ATPS based on tetrahydrofuran (THF) and potassium phosphate buffer for the purification of lipases from Burkholderia cepacia, Candida antarctica, and Aspergillus niger, confirming the potential of the THF-based ATPS for lipase purification [95][18]. The ATPS thus demonstrated its utility as an attractive alternative technique that can meet the greater demands of industrial processes. This technique is also beneficial in terms of economics and environmental sustainability.
3.4. Aqueous Two-Phase Flotation (ATPF)
Bi et al. [96][19] proposed a novel technique to separate and concentrate Penicillin G from a fermentation broth. In this technique, separation by solvent sublation was performed in an aqueous two-phase system and was termed aqueous two-phase flotation (ATPF). Broadly, the ATPF technique is a combination of ATPS and solvent sublation. ATPF is a more effective technique for separating and concentrating biomolecules from an aqueous phase with the reduced consumption of organic solvents. In ATPF, nitrogen gas is passed through the bottom of an enzyme-rich solution which adsorbs the surface-active compounds. Subsequently, the nitrogen bubbles dissolve in the top polymer phase (Figure 4). ATPF is an economical and environmentally friendly technique for the separation and purification of lipases from fermentation broths. However, further studies are required to investigate the optimum rate of flow of nitrogen gas, bubble size, and flotation rate constant for the maximum purification of lipases and other biomolecules.
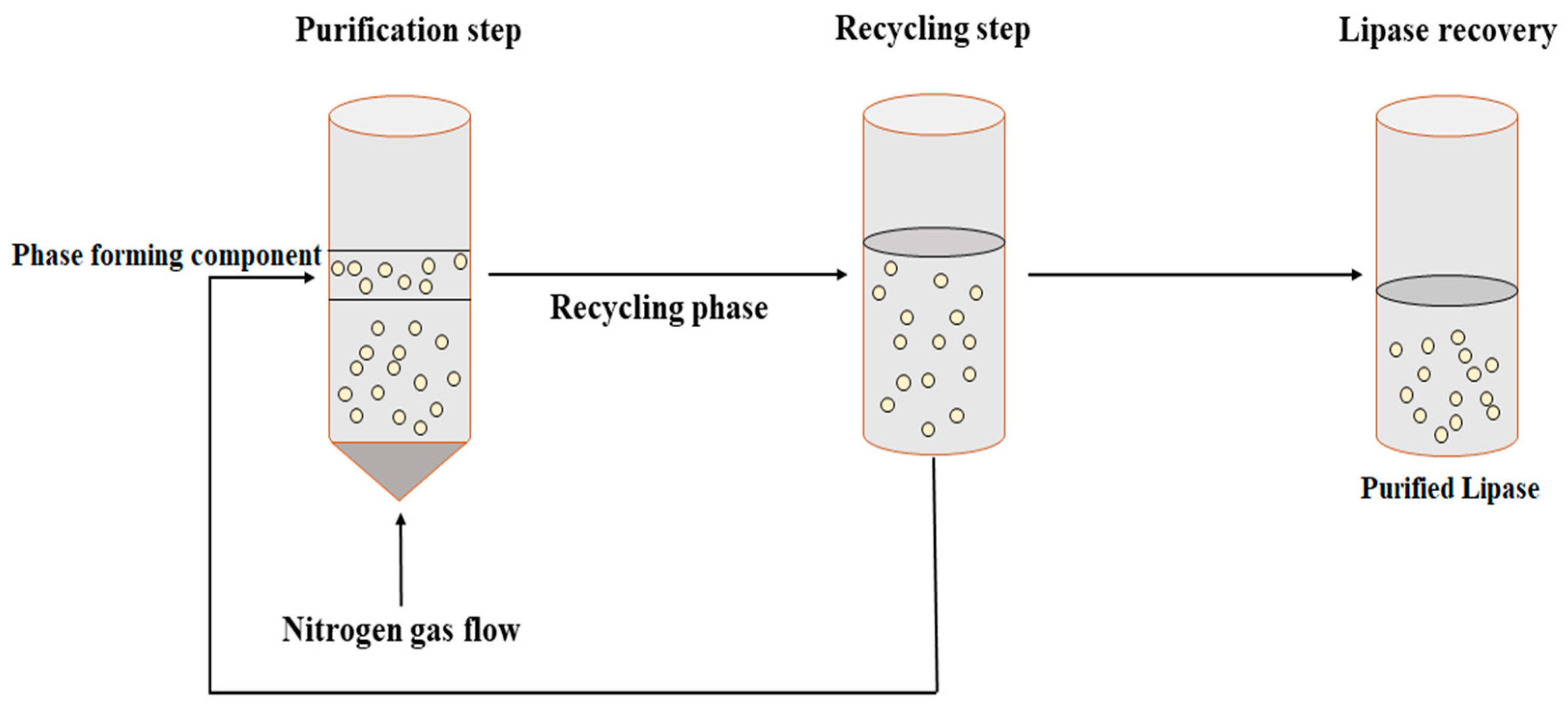
Figure 4.
Schematic presentation of aqueous two-phase flotation (ATPF) for lipase purification.
The use of phase-partitioning chemicals in a conventional ATPS makes it expensive and limits recycling. On the other hand, novel thermoseparating polymers such as ethylene oxide (EO: 50%) and propylene oxide (PO: 50%) (EOPO) can be used [96,97][19][20]. This allows the system to be recycled and achieves higher productivity. Both ATPS and ATPF require a two-step procedure. Initially, the target protein is accumulated and extracted by the top phase of EOPO. This top phase of the initial ATPF results in a secondary phase which separates at a lower critical solution temperature. The top aqueous (water) phase contains the target proteins or biomolecules, while the bottom aqueous phase contains concentrated EOPO solution, which can be reused by recycling for further purification of the target protein. ATPF was used for the direct recovery of lipase derived from Burkholderia cepacia (ST8) from a fermentation broth. The ATPF was composed of EOPO copolymer and ammonium sulfate, wherein the recovery of up to 75% of EOPO and successful purification of the bacterial lipase in a single downstream processing step was reported [97][20].
References
- Saxena, R.K.; Sheoran, A.; Giri, B.; Davidson, W.S. Purification strategies for microbial lipases. J. Microbiol. Methods 2003, 52, 1–18.
- Bharathi, D.; Rajalakshmi, G.; Komathi, S. Optimization and production of lipase enzyme from bacterial strains isolated from petrol spilled soil. J. King Saud Univ.-Sci. 2019, 31, 898–901.
- Ameri, A.; Shakibaie, M.; Faramarzi, M.A.; Ameri, A.; Amirpour-Rostami, S.; Rahimi, H.R.; Forootanfar, H. Thermoalkalophilic lipase from an extremely halophilic bacterial strain Bacillus atrophaeus FSHM2: Purification, biochemical characterization and application. Biocatal. Biotransform. 2017, 35, 151–160.
- Iftikhar, T.; Niaz, M.; Jabeen, R.; Haq, I.U. Purification and characterization of extracellular lipases. Pak. J. Bot 2011, 43, 1541–1545.
- Rahman, M.B.A.; Azaman, R.N.; Omar, E.M.; Latif, M.A.M.; Abdulmalek, E. Candida rugosa lipase immobilized on diethylaminoethyl-cellulose (deae) for esterification of butyl oleate. Malays. J. Anal. Sci. 2019, 23, 376–382.
- Hecht, E.S.; Mehta, S.; Wecksler, A.T.; Aguilar, B.; Swanson, N.; Phung, W.; Dubey Kelsoe, A.; Benner, W.H.; Tesar, D.; Kelley, R.F. Insights into ultra-low affinity lipase-antibody noncovalent complex binding mechanisms. mAbs 2022, 14, 2135183.
- Zhang, S.; Riccardi, C.; Kamen, D.; Reilly, J.; Mattila, J.; Bak, H.; Xiao, H.; Li, N. Identification of the specific causes of polysorbate 20 degradation in monoclonal antibody formulations containing multiple lipases. Pharm. Res. 2022, 39, 75–87.
- Rahimi, H.; Soro, S.; Rughetti, A.; Palocci, C.; Biffoni, M.; Barachini, S.; Taurino, F.; Cernia, E.; Frati, L.; Nuti, M. Monoclonal antibodies against Candida rugosa lipase. J. Mol. Catal. B Enzym. 2004, 28, 71–74.
- Fatima, S.; Faryad, A.; Ataa, A.; Joyia, F.A.; Parvaiz, A. Microbial lipase production: A deep insight into the recent advances of lipase production and purification techniques. Biotechnol. Appl. Biochem. 2021, 68, 445–458.
- Carvalho, C.M.L.; Cabral, J.M.S. Reverse micelles as reaction media for lipases. Biochimie 2000, 82, 1063–1085.
- Behera, S.; Das, S.; Balasubramanian, S. An atomistic view of solvent-free protein liquids: The case of Lipase A. Phys. Chem. Chem. Phys. 2021, 23, 7302–7312.
- Gaikaiwari, R.P.; Wagh, S.A.; Kulkarni, B.D. Efficient lipase purification using reverse micellar extraction. Bioresour. Technol. 2012, 108, 224–230.
- Goswami, D. Lipase Catalysis in Mixed Micelles. ChemBioEng Rev. 2022, 9, 409–418.
- Shehata, M.; Ünlü, A.; Iglesias-Fernández, J.; Osuna, S.; Sezerman, O.U.; Timucin, E. Brave new surfactant world revisited by thermoalkalophilic lipases: Computational insights into the role of SDS as a substrate analog. Phys. Chem. Chem. Phys. 2023, 25, 2234–2247.
- Fernandes, M.L.M.; Krieger, N.; Baron, A.M.; Zamora, P.P.; Ramos, L.P.; Mitchell, D.A. Hydrolysis and synthesis reactions catalysed by Thermomyces lanuginosa lipase in the AOT/Isooctane reversed micellar system. J. Mol. Catal. B Enzym. 2004, 30, 43–49.
- Tan, C.H.; Show, P.L.; Ooi, C.W.; Ng, E.P.; Lan, J.C.W.; Ling, T.C. Novel lipase purification methods–a review of the latest developments. Biotechnol. J. 2015, 10, 31–44.
- Souza, R.L.; Lima, R.A.; Coutinho, J.A.P.; Soares, C.M.F.; Lima, Á.S. Novel aqueous two-phase systems based on tetrahydrofuran and potassium phosphate buffer for purification of lipase. Process Biochem. 2015, 50, 1459–1467.
- Souza, R.L.; Lima, R.A.; Coutinho, J.A.P.; Soares, C.M.F.; Lima, Á.S. Aqueous two-phase systems based on cholinium salts and tetrahydrofuran and their use for lipase purification. Sep. Purif. Technol. 2015, 155, 118–126.
- Bi, P.-y.; Li, D.-q.; Dong, H.-r. A novel technique for the separation and concentration of penicillin G from fermentation broth: Aqueous two-phase flotation. Sep. Purif. Technol. 2009, 69, 205–209.
- Show, P.L.; Tan, C.P.; Anuar, M.S.; Ariff, A.; Yusof, Y.A.; Chen, S.K.; Ling, T.C. Direct recovery of lipase derived from Burkholderia cepacia in recycling aqueous two-phase flotation. Sep. Purif. Technol. 2011, 80, 577–584.
More
