Stroke constitutes the second highest cause of morbidity and mortality worldwide while also impacting the world economy, triggering substantial financial burden in national health systems. High levels of blood glucose, homocysteine, and cholesterol are causative factors for atherothrombosis. These molecules induce erythrocyte dysfunction, which can culminate in atherosclerosis, thrombosis, thrombus stabilization, and post-stroke hypoxia. Glucose, toxic lipids, and homocysteine result in erythrocyte oxidative stress. This leads to phosphatidylserine exposure, promoting phagocytosis. Phagocytosis by endothelial cells, intraplaque macrophages, and vascular smooth muscle cells contribute to the expansion of the atherosclerotic plaque.
- erythrocyte
- ischemic stroke
- atherothrombosis
- lipotoxicity
1. Red Blood Cells Participate in Hypoxia after Atherothrombotic Stroke
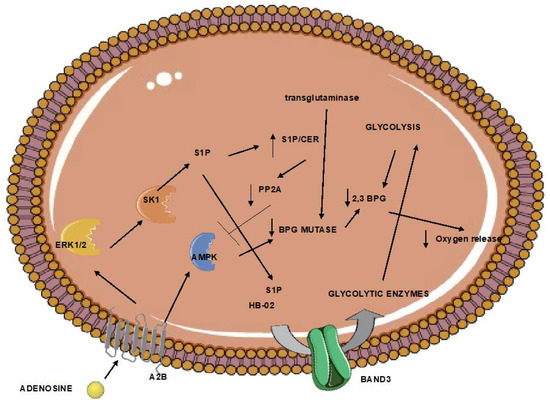
2. Red blood Cells Respond to Damage-Associated Molecular Patterns Released after Ischemic Stroke
| Damp/Pamp | Receptor | Effect |
|---|---|---|
| mtDNA | TLR9 |
|
| CpG DNA | TLR9 |
|
| ATP | P2X7 |
|
| Extracellular histones | TLR2? |
|
| LPS | - |
|
| Amyloids | - |
|
2.1. Cell-Free Mitochondrial DNA and CpG DNA
2.2. ATP
2.3. Extracellular Histones
2.4. Lipopolysaccharides (LPS)
2.5. Amyloids
3. Red Blood Cells Could Connect Non-Alcoholic Fatty Liver Disease with the Risk of Atherothrombotic Strokes
References
- Xu, P.; Chen, C.; Zhang, Y.; Dzieciatkowska, M.; Brown, B.C.; Zhang, W.; Xie, T.; Abdulmalik, O.; Song, A.; Tong, C.; et al. Erythrocyte Transglutaminase-2 Combats Hypoxia and Chronic Kidney Disease by Promoting Oxygen Delivery and Carnitine Homeostasis. Cell Metab. 2022, 34, 299–316.e6.
- Qiang, Q.; Manalo, J.M.; Sun, H.; Zhang, Y.; Song, A.; Wen, A.Q.; Edward Wen, Y.; Chen, C.; Liu, H.; Cui, Y.; et al. Erythrocyte Adenosine A2B Receptor Prevents Cognitive and Auditory Dysfunction by Promoting Hypoxic and Metabolic Reprogramming. PLoS Biol. 2021, 19, e3001239.
- Sun, K.; Zhang, Y.; Bogdanov, M.v.; Wu, H.; Song, A.; Li, J.; Dowhan, W.; Idowu, M.; Juneja, H.S.; Molina, J.G.; et al. Elevated Adenosine Signaling via Adenosine A2B Receptor Induces Normal and Sickle Erythrocyte Sphingosine Kinase 1 Activity. Blood 2015, 125, 1643–1652.
- Sun, K.; Zhang, Y.; D’Alessandro, A.; Nemkov, T.; Song, A.; Wu, H.; Liu, H.; Adebiyi, M.; Huang, A.; Wen, Y.E.; et al. Sphingosine-1-Phosphate Promotes Erythrocyte Glycolysis and Oxygen Release for Adaptation to High-Altitude Hypoxia. Nat. Commun. 2016, 7, 12086.
- Kimura, H.; Hamasaki, N.; Yamamoto, M.; Tomonaga, M. Circulation of Red Blood Cells Having High Levels of 2,3-Bisphosphoglycerate Protects Rat Brain from Ischemic Metabolic Changes during Hemodilution. Stroke 1995, 26, 1431–1437.
- Domingo-Ortí, I.; Lamas-Domingo, R.; Ciudin, A.; Hernández, C.; Herance, J.R.; Palomino-Schätzlein, M.; Pineda-Lucena, A. Metabolic Footprint of Aging and Obesity in Red Blood Cells. Aging 2021, 13, 4850.
- Stanzione, R.; Forte, M.; Cotugno, M.; Bianchi, F.; Marchitti, S.; Rubattu, S. Role of DAMPs and of Leukocytes Infiltration in Ischemic Stroke: Insights from Animal Models and Translation to the Human Disease. Cell. Mol. Neurobiol. 2022, 42, 545–556.
- Hakoupian, M.; Ferino, E.; Jickling, G.C.; Amini, H.; Stamova, B.; Ander, B.P.; Alomar, N.; Sharp, F.R.; Zhan, X. Bacterial lipopolysaccharide is associated with stroke. Sci. Rep. 2021, 11, 6570.
- Pfefferlé, M.; Ingoglia, G.; Schaer, C.A.; Yalamanoglu, A.; Buzzi, R.; Dubach, I.L.; Tan, G.; López-Cano, E.Y.; Schulthess, N.; Hansen, K.; et al. Hemolysis transforms liver macrophages into antiinflammatory erythrophagocytes. J. Clin. Investig. 2020, 130, 5576–5590.
- Ni, W.; Mao, S.; Xi, G.; Keep, R.F.; Hua, Y. Role of Erythrocyte CD47 in Intracerebral Hematoma Clearance. Stroke 2016, 47, 505–511.
- Hotz, M.J.; Qing, D.; Shashaty, M.G.S.; Zhang, P.; Faust, H.; Sondheimer, N.; Rivella, S.; Worthen, G.S.; Mangalmurti, N.S. Red Blood Cells Homeostatically Bind Mitochondrial DNA through TLR9 to Maintain Quiescence and to Prevent Lung Injury. Am. J. Respir. Crit. Care Med. 2018, 197, 470–480.
- Lam, L.K.M.; Murphy, S.; Kokkinaki, D.; Venosa, A.; Sherrill-Mix, S.; Casu, C.; Rivella, S.; Weiner, A.; Park, J.; Shin, S.; et al. DNA binding to TLR9 expressed by red blood cells promotes innate immune activation and anemia. Sci. Transl. Med. 2021, 13, eabj1008.
- Parker, J.C.; Snow, R.L. Influence of external ATP on permeability and metabolism of dog red blood cells. Am. J. Physiol. 1972, 223, 888–893.
- Sluyter, R.; Shemon, A.N.; Barden, J.; Wiley, J.S. Extracellular ATP increases cation fluxes in human erythrocytes by activation of the P2X7 receptor. J. Biol. Chem. 2004, 279, 44749–44755.
- Sluyter, R.; Shemon, A.N.; Hughes, W.E.; Stevenson, R.O.; Georgiou, J.G.; Eslick, G.D.; Taylor, R.M.; Wiley, J.S. Canine erythrocytes express the P2X7 receptor: Greatly increased function compared with human erythrocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, 2090–2098.
- Faulks, M.; Kuit, T.A.; Sophocleous, R.A.; Curtis, B.L.; Curtis, S.J.; Jurak, L.M.; Sluyter, R. P2X7 receptor activation causes phosphatidylserine exposure in canine erythrocytes. World J. Hematol. 2016, 5, 88–93.
- Sophocleous, R.A.; Mullany, P.R.F.; Winter, K.M.; Marks, D.C.; Sluyter, R. Propensity of red blood cells to undergo P2X7 receptor-mediated phosphatidylserine exposure does not alter during in vivo or ex vivo aging. Transfusion 2015, 55, 1946–1954.
- Semeraro, F.; Ammollo, C.T.; Esmon, N.L.; Esmon, C.T. Histones induce phosphatidylserine exposure and a procoagulant phenotype in human red blood cells. J. Thromb. Haemost. 2014, 12, 1697–1702.
- Kordbacheh, F.; O’Meara, C.H.; Coupland, L.A.; Lelliott, P.M.; Parish, C.R. Extracellular histones induce erythrocyte fragility and anemia. Blood 2017, 130, 2884–2888.
- Yeung, K.W.; Lau, P.M.; Tsang, H.L.; Ho, H.P.; Kwan, Y.W.; Kong, S.K. Extracellular histones induced eryptotic death in human erythrocytes. Cell. Physiol. Biochem. 2019, 53, 229–241.
- Noubouossie, D.; Sokol, J.; Piegore, M.G.; Ilich, A.; Henderson, M.W.; Mooberry, M.; Monroe, D.; Key, S.N. Histones Induce the Release of Extracellular Hemoglobin and Red Blood Cell-Derived Microvesicles with Procoagulant Activity. Blood 2018, 132 (Suppl. S1), 2514.
- Gwoździński, K.; Pienia̧zek, A.; Kaca, W. Lipopolysaccharide from Proteus mirabilis O29 induces changes in red blood cell membrane lipids and proteins. Int. J. Biochem. Cell Biol. 2003, 35, 333–338.
- Gwozdzinski, K.; Pieniazek, A.; Sudak, B.; Kaca, W. Alterations in human red blood cell membrane properties induced by the lipopolysaccharide from Proteus mirabilis S1959. Chem. Biol. Interact. 2003, 146, 73–80.
- Brauckmann, S.; Effenberger-Neidnicht, K.; De Groot, H.; Nagel, M.; Mayer, C.; Peters, J.; Hartmann, M. Lipopolysaccharide-induced hemolysis: Evidence for direct membrane interactions. Sci. Rep. 2016, 6, 35508.
- Nicolay, J.P.; Gatz, S.; Liebig, G.; Gulbins, E.; Lang, F. Amyloid induced suicidal erythrocyte death. Cell. Physiol. Biochem. 2007, 19, 175–184.
- Tikhonova, L.A.; Kaminskii, Y.G.; Kosenko, E.A. Effects of amyloid-β peptide Aβ25-35 on glycolytic and antioxidant enzymes in erythrocytes of different ages. Biol. Bull. 2014, 41, 312–317.
- Clementi, M.E.; Giardina, B.; Colucci, D.; Galtieri, A.; Misiti, F. Amyloid-beta peptide affects the oxygen dependence of erythrocyte metabolism: A role for caspase 3. Int. J. Biochem. Cell Biol. 2007, 39, 727–735.
- Misiti, F.; Orsini, F.; Clementi, M.E.; Masala, D.; Tellone, E.; Galtieri, A.; Giardina, B. Amyloid peptide inhibits ATP release from human erythrocytes. Biochem. Cell Biol. 2008, 86, 501–508.
- Carelli-Alinovi, C.; Giardina, B.; Misiti, F. Amyloid beta peptide (1-42)-mediated antioxidant imbalance is associated with activation of protein kinase C in red blood cells. Cell Biochem. Funct. 2015, 33, 196–201.
- Carelli-Alinovi, C.; Dinarelli, S.; Sampaolese, B.; Misiti, F.; Girasole, M. Morphological changes induced in erythrocyte by amyloid beta peptide and glucose depletion: A combined atomic force microscopy and biochemical study. Biochim. Biophys. Acta-Biomembr. 2019, 1861, 236–244.
- Xu, J.; Dai, L.; Zhang, Y.; Wang, A.; Li, H.; Wang, Y.; Meng, X.; Wu, S.; Wang, Y. Severity of Nonalcoholic Fatty Liver Disease and Risk of Future Ischemic Stroke Events. Stroke 2021, 52, 103–110.
- Papadopoulos, C.; Spourita, E.; Mimidis, K.; Kolios, G.; Tentes, L.; Anagnostopoulos, K. Nonalcoholic Fatty Liver Disease Patients Exhibit Reduced CD47 and Increased Sphingosine, Cholesterol, and Monocyte Chemoattractant Protein-1 Levels in the Erythrocyte Membranes. Metab. Syndr. Relat. Disord. 2022, 20, 377–383.
- Papadopoulos, C.; Mimidis, K.; Tentes, I.; Tente, T.; Anagnostopoulos, K. Validation and application of a protocol for the extraction and quantitative analysis of sphingomyelin in erythrocyte membranes of patients with non-alcoholic fatty liver disease. JPC-J. Planar Chromatogr. 2021, 34, 411–418.
- Papadopoulos, C.; Mimidis, K.; Papazoglou, D.; Kolios, G.; Tentes, I.; Anagnostopoulos, K. Red Blood Cell-Conditioned Media from Non-Alcoholic Fatty Liver Disease Patients Contain Increased MCP1 and Induce TNF-α Release. Rep. Biochem. Mol. Biol. 2022, 11, 54–62.
- Otogawa, K.; Kinoshita, K.; Fujii, H.; Sakabe, M.; Shiga, R.; Nakatani, K.; Ikeda, K.; Nakajima, Y.; Ikura, Y.; Ueda, M.; et al. Erythrophagocytosis by liver macrophages (Kupffer cells) promotes oxidative stress, inflammation, and fibrosis in a rabbit model of steatohepatitis: Implications for the pathogenesis of human nonalcoholic steatohepatitis. Am. J. Pathol. 2007, 170, 967–980.
