Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Martina Catalano and Version 2 by Sirius Huang.
Neoplastic as well as surrounding stromal and inflammatory cells engage in well-orchestrated reciprocal interactions to establish an inflammatory tumor microenvironment. The tumor-associated inflammatory tissue is highly plastic, capable of continuously modifying its phenotypic and functional characteristics. Accumulating evidence suggests that chronic inflammation plays a critical role in the development of urological cancers.
- inflammation
- tumorigenesis
- cancer
- genitourinary tumors
1. Introduction
Although inflammation is a self-limiting host defense strategy against biological, chemical, and physical agents, it is also considered a hallmark of cancer development. Inflammation, especially if persistent, stimulates cell proliferation and local host response, causing cell damage and the development of various diseases including cancer [1][2][1,2]. In addition, the tumor microenvironment (TME), enriched in cytokines, chemokines, transcription factors, and immune cells, can enhance tumor growth and immune escape. Inflammation is an important participant in the narrative regarding tumors, and oncogenesis is estimated to correlate with chronic infection and inflammation in 15–20% of cancers [3]. Etiological factors such as Helicobacter pylori, hepatitis B or C, and autoimmune diseases, are linked to gastric and colorectal cancer, hepatocellular carcinoma, and mucosa-associated lymphoid tissue lymphoma [4][5][4,5].
Over the last few years, numerous studies have shown that inflammatory molecules and pathways promote the development of various cancer types, including genitourinary tumors [6][7][8][6,7,8]. In bladder cancer (BC), chronic inflammation (e.g., urogenital schistosomiasis) is accepted as a risk factor together with other well-established causes such as smoking and occupational exposure to aromatic amines [9]. Advanced age, African descent, family history, and genetic mutations are known to be risk factors for prostate cancer (PCa), but the mechanisms responsible for initiation and progression have not yet been fully elucidated [10][11][10,11]. Inflammation-induced cellular stress and repeated genomic damage have been investigated as possible causes of PCa [12][13][12,13]. Accumulating evidence shows that immune cells and inflammatory pathways can promote renal cell carcinoma (RCC) growth and immune escape [14], whereas there are insufficient grounds to claim a role for inflammation in other tumors of the urogenital tract, such as testicular and penile cancer.
2. Inflammation as Risk Factor in Genitourinary Cancers
Inflammatory response appears to be fundamental in the occurrence and development of tumors, however, its role in carcinogenesis still requires further clarification [12]. Several factors, such as pathogens, diet, mechanical and chemical trauma, are able to initiate an inflammatory process. Two different models have been proposed to describe how inflammation is related to cancer: (1) an intrinsic pathway induced by DNA damage, chromosomal instability, and epigenetic changes; and (2) an extrinsic pathway associated with inflammatory signals caused by autoimmune diseases or infections [15]. Both these pathways are characterized by the activation of transcription factors, such as Nuclear Factor-κB (NF-κB) and Signal Transducer and Activator of Transcription (STAT)-3, which drive the inflammatory cascade [16][17][16,17]. Overall, the most frequent causes of genitourinary inflammation reside in infectious and noninfectious etiology (Figure 1).
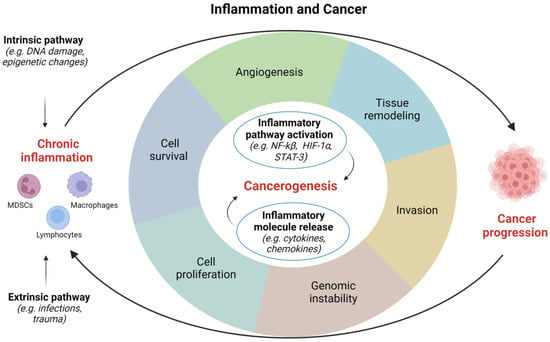
Figure 1. Correlations between inflammation and cancer. HIF, hypoxia inducible factor; NF, nuclear factor; STAT, signal transducer and activator of transcription. Image created with BioRender.com (accessed on 1 December 2022).
2.1. Infectious Agents
2.1.1. Parasites
Schistosomiasis is a recognized risk factor for BC, the most common cancer type in men and the second in women in endemic regions of Sudan, Egypt, sub-Saharan Africa, and Yemen [18]. Squamous cell carcinomas constitute 60–90% and adenocarcinomas 5–15% of all schistosomiasis-associated BC, the rest being urothelial carcinomas [19]. Schistosoma haematobium eggs stimulate an inflammatory response often generating genotoxic factors that determine genomic instability and tissue damage. In vivo studies in CD-1 mice have shown that intravesical instillation of Schistosoma haematobium antigens causes inflammation and urothelial dysplasia [20]. It has been hypothesized that Schistosoma haematobium is oncogenic by inducing K-RAS mutations [21]. To combat helminthic infection, inflammatory cells release reactive oxygen species (ROS) and reactive nitrogen species which, chronically released at low concentrations, function as intra- and intercellular second messengers regulating cell proliferation, apoptosis, and gene expression [22][23][22,23].
2.1.2. Bacterial Infections
The correlation between bacterial infections and the development of BC is still controversial. Some authors have reported a greater risk of BC in patients with a history of recurrent (three or more within 12 months) urinary tract infections (UTIs), whereas fewer UTI events treated with antibiotics correlate to a lower risk of BC [24][25][24,25]. Conversely, Jiang et al. demonstrated a significantly reduced risk of BC in patients with recurrent UTIs, possibly explained by the anti-cancer effect of the antimicrobial treatment, higher exposure to non-steroidal anti-inflammatory drugs, and immune response induced by bladder infection [26].
Gram-negative uropathogens are the main pathogens involved in bacterial prostatitis, however, Gram-positive and atypical microorganisms can also be causative agents [27]. Bacterial protein toxins could function as carcinogenetic stimuli, damaging DNA directly via enzymatic attack, indirectly by stimulating an inflammatory reaction that generates free radicals, or else by interfering with DNA repair mechanisms [28][29][28,29].
2.1.3. Viruses
High-risk human papillomavirus (HPV) infection is a key factor in the development of several tumors, including penile carcinoma [30]. Cells harboring HPV genomes display increased levels of nitric oxide (NO), which fuels inflammation and enhances DNA damage [31]. The HPV genome contains genes encoding for E7 oncoprotein, which inactivates the retinoblastoma (Rb) tumor suppressor gene normally suppressing p16, and consequently high-level p16 expression is useful as a surrogate marker for HPV infection.
A thorough understanding of the role of HPV in bladder carcinogenesis has not been reached. Some authors have identified HPV as a causal factor [32][33][32,33] but this has not been confirmed by others [34][35][34,35].
HPV infection as a potential cause of PCa is a matter of debate. Since the prevalence of high-risk HPV in benign prostate tissue is similar to that observed in PCa samples, it could be sustained that the presence of HPV is coincidental. However, a systematic review by Lawson and Glenn has lately established that high-risk HPV types are more common in PCa than in benign tissue [36]. This finding is confirmed by a recent meta-analysis suggesting that HPV infection is related to an increased likelihood of PCa development, the odds ratio being 2.27 (95% CI, 1.40–3.69) [37].
Regarding hepatitis C virus (HCV) infection, recent data show a correlation between HCV infection and a greater risk of RCC. Conversely, this correlation is not statistically significant for PCa and BC [38].
Several authors have discussed the role of specific viruses in the development of testicular germ cell tumors (TGCTs). While high-quality evidence indicates that human immunodeficiency virus (HIV) and EBV infection increase the risk of TGCTs, results on the relationship with cytomegalovirus (CMV) and HPV are inconsistent, calling for further studies to draw firm conclusions [39].
2.2. Noninfectious Causes
The inflammatory process in the urogenital tract can be prompted by causes other than infectious. Local mechanical disorders may provoke metaplastic changes of the urothelium (e.g., cystitis cystica et glandularis, intestinal metaplasia, and nephrogenic adenoma) or lesions that carry a risk of progression to malignancy (e.g., keratinizing squamous metaplasia) [40].
Spinal cord injury causing neurogenic bladder and chronic indwelling catheter usage have been identified as risk factors for BC, but literature data are conflicting. Although a higher risk of BC has been reported in patients with spinal cord injury than in the general population [41][42][41,42], other evidence shows that the risk of squamous cell carcinoma (SCC) of the urinary bladder increases in patients with spinal cord injury requiring indwelling catheters (42%) rather than other types of catheterization (e.g., clean intermittent, condoms) or spontaneous urination [43]. Some authors have shown that, after adjustment for sex, age and comorbidities, any difference in BC incidence between patients with spinal cord injury and controls is negligible [44].
Regarding prostate inflammation, much attention has been focused on the role of diet, smoking, changes in serum testosterone and estrogen levels, autoimmunity, reflux of noxious chemicals in the urine, and metabolic alterations [8]. A high-fat diet correlates with a significant increase in pro-inflammatory cytokines and activation of pathways (e.g., STAT3 and NF-κB) involved in the proliferation, survival, angiogenesis, and invasion of prostate neoplastic cells [45]. In addition, studies in animal models have determined that estrogens can modulate the immune response and spur prostatitis [46][47][46,47]. Rats with both spontaneous and sex hormone-induced prostatitis display a reduced proportion of intraprostatic NK-like cells and a low ratio of NKT/T cells [48]. Significantly higher rates of proinflammatory cytokines, such as tumor necrosis factor (TNF)-α and macrophage inflammatory protein (MIP)-1α, have been found in estrogen-treated rats than in normal and castrated controls [49]. Despite this, the exact mechanisms of estrogen-mediated immunomodulation remain unclear.
