Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Rifat Mehdi.
Biochar (BC) based materials are solid carbon enriched materials produced via different thermochemical techniques such as pyrolysis.
- biomass
- pyrolysis
- biochar production
1. Introduction
Increasing population, energy demands, and depletion of natural non-renewable energy resources like coal, gas, and petroleum threaten the energy security of the planet [1]. It is very important to develop cheap and good environmental solutions to address these energy-related problems. In this regard, energy storage and conversion from renewables like biomass can be the right alternate to solve energy requirements [2,3][2][3]. For energy storage, there is the development of low cost material to be synthesized from the abundantly available renewables and natural resources like lignocellulosic biomasses [4].
Sustainable energy generation requires efficient energy storage equipment to harvest energy [5]. Among those storage equipment, lithium-ion batteries attracted much attention due to their high energy density. Short life, low power density, and high cost are the issues associated with lithium-ion batteries [6]. Therefore, it is required to develop a device with better energy storage performance. Supercapacitors gained interest for being a promising energy storage device for the future due to their fast charge/discharge rate, high power density, and good cycle stability [7]. However, due to their lower energy density, they are not widely used in the energy storage market. Researchers are working to develop such energy storage materials to achieve promising performance in energy applications [8]. In order to evaluate the performance of the supercapacitor, the basic elements related to the electrodes and electrolyte are considered [9]. The capacitive performance of electrodes are highly desirable and can be increased with the help of stable, long lasting, and electrically conductive electrode material. These properties along with surface wettability are of prime importance while fabricating the electrode materials [10]. Carbon materials obtained from biomass have been considered as suitable materials for supercapacitors because of their physicochemical properties like high surface area, high porosity, electrical conductivity, functional groups, availability of metal ions and minerals, stable structure against electrochemical activity, and their morphology for ions mobility [11,12][11][12].
Lignocellulosic biomass provide the chemically stable, tunable pore structure, and high surface area carbonaceous material. Due to these properties, the lignocellulosic biomass is advantageous over the other material for the application of high electrical conductance [13]. The carbon materials produced from biomass have attracted much attention due to their environmental friendly nature, natural abundance, and special porous features in energy storage and conversion applications [14]. Biochar (BC)-based materials played a vital role in energy storage and conversion, including making electrodes for supercapacitors [15], electro-catalysts [16], and lithium-ion batteries. Conventional carbon materials mainly obtained from the non-renewable sources require severe production conditions and synthesis method [17]. Hence, in order to adopt environment friendly methods of producing carbon-based material, a sustainable source of raw material needs to be adopted for positive prospects. In order to produce BC-based material, the promising raw material can be the biomass that has an inherent potential content of 45 to 50 wt% carbon [18]. Lignocellulosic biomass is composed of cellulose (40–50 wt%), hemicelluloses (15–30 wt%), and lignin (15–30 wt%) [19]. The composition of biomass indicates the decomposition of contents at temperatures that are lower than 500 °C. This feature exists due to the presence of lignin, cellulose, and the hemicellulose in biomass. This biomass can easily be decomposed to produce BC, bio-oil, and incondensable gases, which are a direct result of pyrolysis at low temperature. However, BC materials show poor capacity retention and lower specific capacitance due to poor pore features and lower surface area. The BC materials with pre- or post-treatment show promising results of electrochemical applications. A higher pyrolysis temperature and treated BC can present the correct pore properties and electrochemical applications [20]. Inadequate pore structure and poor size distribution in BC-based materials greatly affect the performance of supercapacitors. These green pyrolyzed biomass materials exhibit an amorphous structure, which lowers their electrical conductivity and also affects the performance of supercapacitors. Due to the carbonization temperature, aromatic carbon contents increase with the significant loss of surface functionalities like carboxyl, carbonyl, and hydroxyl [21]. BC, having physicochemical properties like high porosity, functional groups, superior stability, a large surface area, availability of metal cation and minerals, and high cation exchange capacity can be a promising candidate for energy and environmental applications [12]. These physicochemical properties can be modified according to the required electrochemical applications. Therefore, to tailor the structure of the BC, surface functionalities and activation should be introduced by different surface modification methods to obtain an excellent energy storage material for supercapacitors.
Previously different modifications were reviewed separately. Babasaheb M. Mastagar et al. [22] reviewed the biomass-derived nitrogen-doped porous carbon and its various applications like catalysis and electrochemical energy storage. Baharak Sajjadi et al. [23,24][23][24] presented a review article sttudying the physical action and chemical activation of BC for environment and energy sectors. Wei-Hao Huang et al. [25] updated the research efforts for BC modifications, particularly to the end applications based of physical and chemical activation. Feng Shen et al. [26] updated mechanisms that are employed in order to value add the carbon materials in the context of mechano-chemical conversion. Emanuela Calcio Gaudino et al. [27] studied the sono-chemical and mechano-chemical surface modification techniques to valorize the biomass. However, there is no review that discusses the surface modification techniques to produce surface modified BC-based material with desired surface features to achieve the excellent performance in supercapacitors application. Therefore, the objective of this work is to provide a state of the art review on BC production via pyrolysis, surface modification, and application for supercapacitors to store energy.
2. Overview of Biomass Pyrolysis for BC Production
Pyrolysis is one of the thermo-chemical conversion methods used to decompose biomass into useful products in a limited supply of oxygen or in an inert environment that does not allow gasification to some extent. Pyrolysis is carried out at temperature ranging from 300–650 °C for a specific time interval to produce non-condensable gases, solid char, and liquid products [28]. BC is a solid carbonaceous material produced via different thermal techniques along with liquid and gas as valuable products from widely available biomass feedstocks. During BC production via pyrolysis, biomass undergoes a set of complex reactions. To restructure the morphology, porosity, and functionality of the biomass-derived BC, it is very important to understand these reactions [29]. In pyrolysis the degrees of polymerization is an important factor in the reaction mechanism of pyrolysis [30]. These reactions are affected by key constituents of the employed biomass, which include lignin, cellulose, and hemicellulose. During pyrolysis, these components decompose at different temperature zones for disintegration to produce the porous carbonaceous material. The pyrolysis process has successive stages for the conversion and disintegration of biomass. The initial process is the removal of the absorbed moisture, which takes place at a temperature of about 100–150 °C, and the degradation of hemicellulose that occurs from 150–260 °C. Afterwards, the cellulosic decomposition range is from 240–350 °C, and finally, in the case of lignin, the decomposition takes place at about 280–500 °C [28]. Thus, during pyrolysis process de-volatilization of volatiles–which constitutes of carbon monoxide, carbon dioxide, and methane–takes place to produce BC and black solid, which is majorly solid carbon. As biomass has complex physical and chemical properties and structure, the produced carbonaceous material possesses poor surface structure. However, these carbon materials can be employed for different applications like batteries, capacitors, and soil and environmental remediation. Consequently, these resultant BC-based materials require surface modification for activation, which need to bear the controlled attributes of surface chemistry, porosity, surface area, and stability. Primarily, the biomass has cellulose, hemicellulose, and lignin as constituents. These components of biomass provide a strong rigid structure to plants due to chemical bonds [31]. Pyrolysis of biomass is performed under an inert atmosphere in the temperature range of 350–700 °C [32]. The operating conditions such as heating rate, temperature, and residence time greatly influence the distribution of the products. A lower heating rate, long residence time, and lower pyrolysis temperature (slow pyrolysis) favors the production of char, while a relatively higher temperature, short residence time, and higher heating rate (fast pyrolysis) promotes the oil yield [33]. However, the increased temperature could lead to cracking of the volatile, thereby enhancing the yield of on-condensable gases. The operating conditions in pyrolysis experiments can be optimized depending on the desired products. Effective energy storage applications can be manifested by producing BC from biomass, employing the pyrolysis process and modifying the surface via activation. This modification is an efficient approach to store energy. BC-based materials have well developed pore structure and functional groups, hence the selection of correct material is important in order to employ the precursor to obtain the desired attributes for enhanced applicability. Biomass precursors for BC production should be cheap, abundant, renewable, diverse, and environment friendly. To obtain the desired surface and textural properties, higher fixed carbon is imperative in the biomass along with the lowest possible ash contents [34]. A vast variety of biomass is available, such as woody and non-woody biomass, industrial waste biomass, and agricultural biomass wastes to be considered for the selection of the precursor with which to produce BC-based materials. The physicochemical properties of biomass for BC-based material production are based on elemental composition, proximate analysis, particle size, grindability, and density of the biomass. Lignocellulosic biomass contains mainly carbon, hydrogen, oxygen, nitrogen, calcium, potassium, and minor concentration of Si, P, Al, Mg, and Fe, etc. Due to some of the constraints like high moisture content, low bulk density, diverse physicochemical properties, and seasonal variation, lignocellulosic biomasses are not used as a direct energy conversion source [35]. For thermochemical conversion to make biochar, it is necessary to understand the chemical composition of biomass. The components in lignocellulosic biomass such as hemicellulose, cellulose, and lignin have certain decomposition temperature and degradation rates. Hemicellulose decomposition starts earlier than for cellulose and lignin. Generally, hemicellulose produces lower char, tar, and gas [36,37][36][37], while lignin produces higher char and less gases compared to cellulose. Cellulose has been reported to produce higher oil yield and lower gases and char yield [36,37][36][37]. Cotton stalk has lower hemicellulose (27.98%) and lignin (20.51%) contents, which promotes less char yield, while the cellulose content of 40.17% promotes a higher production of oil [38]. The hemicellulose and lignin content of biomass are critical compositional parameters for char production. The feedstocks with higher lignin content have been reported to produce higher char yield compared to those with higher hemicellulose content. Khan et al. [39] investigated the pyrolysis of rice straw and waste tire at 550 °C in a fixed bed reactor. The char yield from the pyrolysis of rice straw alone was slightly lower than the oil yield, which corresponds to the lower lignin content. Moreover, Ma et al. [40] reported that the char obtained from lignin is superior to char derived from hemicellulose in the terms of mass and energy yield. However, the surface structure and porosity of the char derived from cellulose is superior compared to that of lignin. Lignin, due to its complex structure, degrades at a wide range of temperature ranging from 150–900 °C, and has a complex degradation mechanism that causes a lower surface area and total pore volume [41]. It was also found that the higher the lignin content, the higher the surface area and porosity, which is due to the stable structure of lignin and preserved pore structure [42,43][42][43]. Similarly, the feedstocks with higher fixed carbon content will produce a higher char yield compared to those with higher volatile contents. The ash content is another compositional parameter of feedstock that significantly impacts the quality of char. Char with a lower ash content renders higher porosity, and a higher ash content causes a lower surface area as ash blocks the pores of biochar, which restricts its performance in supercapacitor applications [44]. Figure 1 shows factors affecting the pyrolysis process, subsequently on the physicochemical and electrical properties of BC. For instance, various factors have been studies and found a great impact on the properties and production of BC, which include the reaction temperature, heating rate, and nature of the feedstock as major influencers [45]. The composition of biomass has a promising impact on the yield of the pyrolysis process, especially its hydrogen-to-carbon (H/C) and oxygen-to-carbon (O/C) ratio. With components of lignocellulosic biomass, each one has its own decomposition temperature range like hemicellulose (150–350 °C), cellulose (275–350 °C), and lignin (250–500 °C) [46]. The particle size of the biomass feedstock has an effect on the products of the pyrolysis process. The smaller the particle size, the higher the liquid yield, as it offers less resistance to the release of condensable gases. On the other hand, a bigger particle size resists the primary product of pyrolysis, which favours secondary cracking to yield solid product. The pyrolysis reaction temperature is the parameter that can affect yield, as well as physical and chemical properties of the BC. Previously, achnatherum was pyrolyzed at 300 °C, 500 °C, and 700 °C, which enunciated a decrease in BC yield from 48% to 24% with an increasing reaction temperature, which further resulted in the gradual disappearance of the functional groups on the BC [47]. Pyrolysis temperature has a prominent impact of the surface area and total pore volume. At lower temperatures of less than 400 °C, only a slight change occurs as it does not provide appropriate conditions for complete devolatilization, which restricts the formation of new pores [48]. As the temperature rises, the amorphous carbon changes to crystalline and higher temperature also provides activation energy, which leads to micropore formation [49]. The heating rate has a remarkable effect on the surface features and yield of the BC. BC yield is increased at low heating rates because the decomposition of the biomass is lowered during the secondary cracking process. The formation of aromatic structures in BC occurs at lower heating rates rather than at a fast heating rate [50]. The heating rate has a very close relation to BC-specific surface area and porosity. For instance, when the heating rate was increased from 1 °C/min to 20 °C/min, the surface area of rapeseed stem-derived BC was increased from 295.9 m2/g to 384.1 m2/g, while the pore volume was increased from 0.1659 cm3/g to 0.2192 cm3/g [51]. In another study, it was found that when the heating rate was increased from 10 °C/min to 30 °C/min, the surface area was found to be increased from 210.4 m2/g to 411.06 m2/g, but it was decreased to 385.38 m2/g when the heating rate was further increased to 50 °C/min [52]. From the previous studies, a conclusion can be drawn that a suitable and preferable surface area and porosity of biochar can be achieved at heating rate from 5 °C/min to 30 °C/min. Residence time also has a great influence on the surface area and porosity of the BC. In addition, it was also studied that surface area of rapeseed stem-derived BC increased from 46.7 to 98.4 m2/g against the residence time increasing from 10 min to 60 min, but the surface area decreased to 91.4 m2/g when the residence went to 100 min. Prolonged residence time and exposure to high temperature leads to the destruction of pores, therefore, a residence time in the range of 30–120 min is generally considered appropriate [51]. During pyrolysis, nitrogen gas is generally used, which has an important impact on the surface properties of BC. The surface area and pore volume increase with the increase in the flow rate of N2 gas during pyrolysis. Previous study shows that the surface area of BC increased from 36 m2/g to 352 m2/g against the respective gas flow rates of 50 to 150 mL/min [53]. Devolatilization increases with the increased flow of gas, which causes pore formation to increase the surface area [54]; however, a flow rate that is too high leads to less release of volatile matter, which indicates a lower surface area and pore volume [53].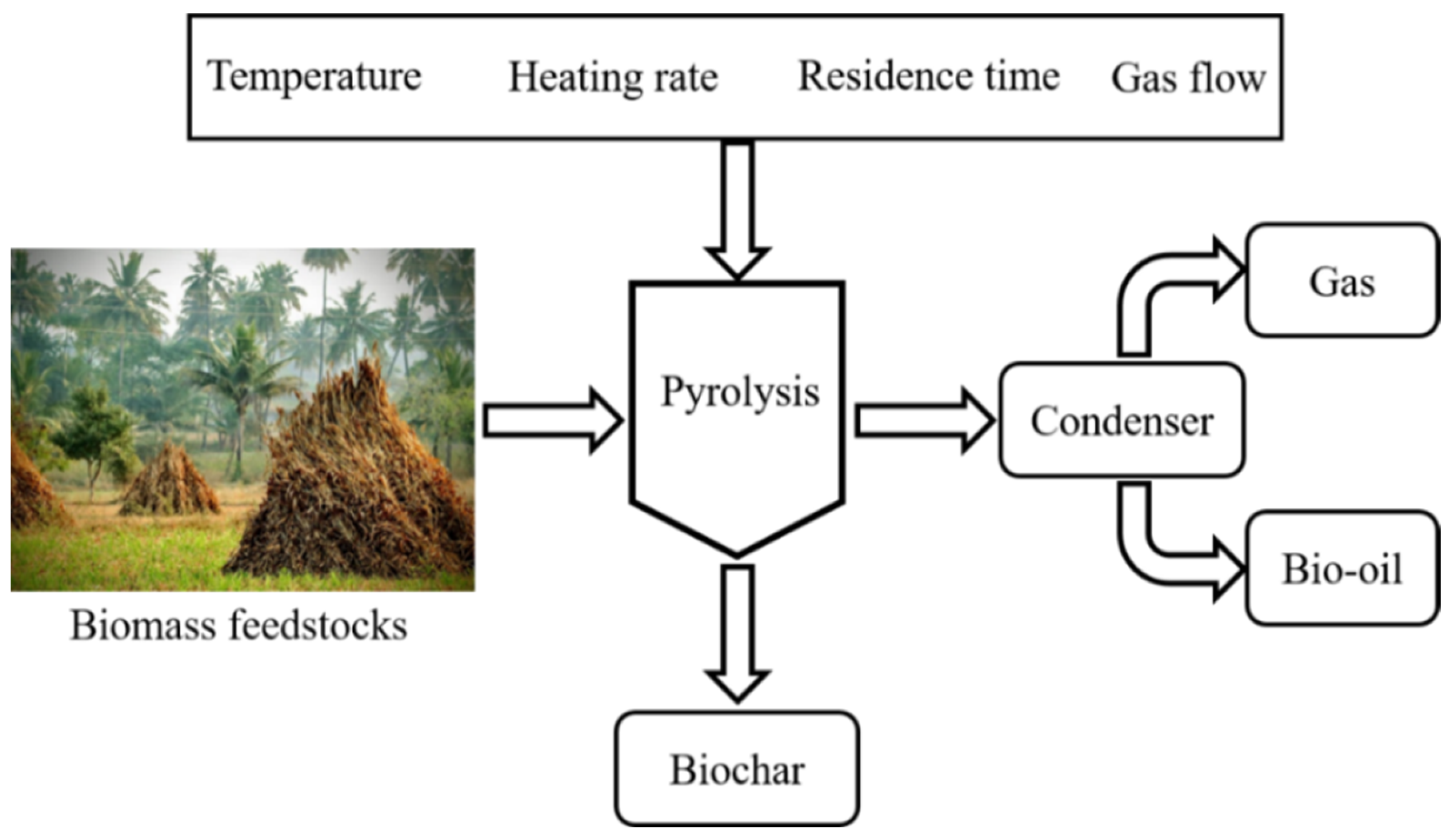
Figure 1. Factors affecting the pyrolysis process of biomass. Recreated with permission from Ref. [55]. Copyright © 2022 Springer Nature.
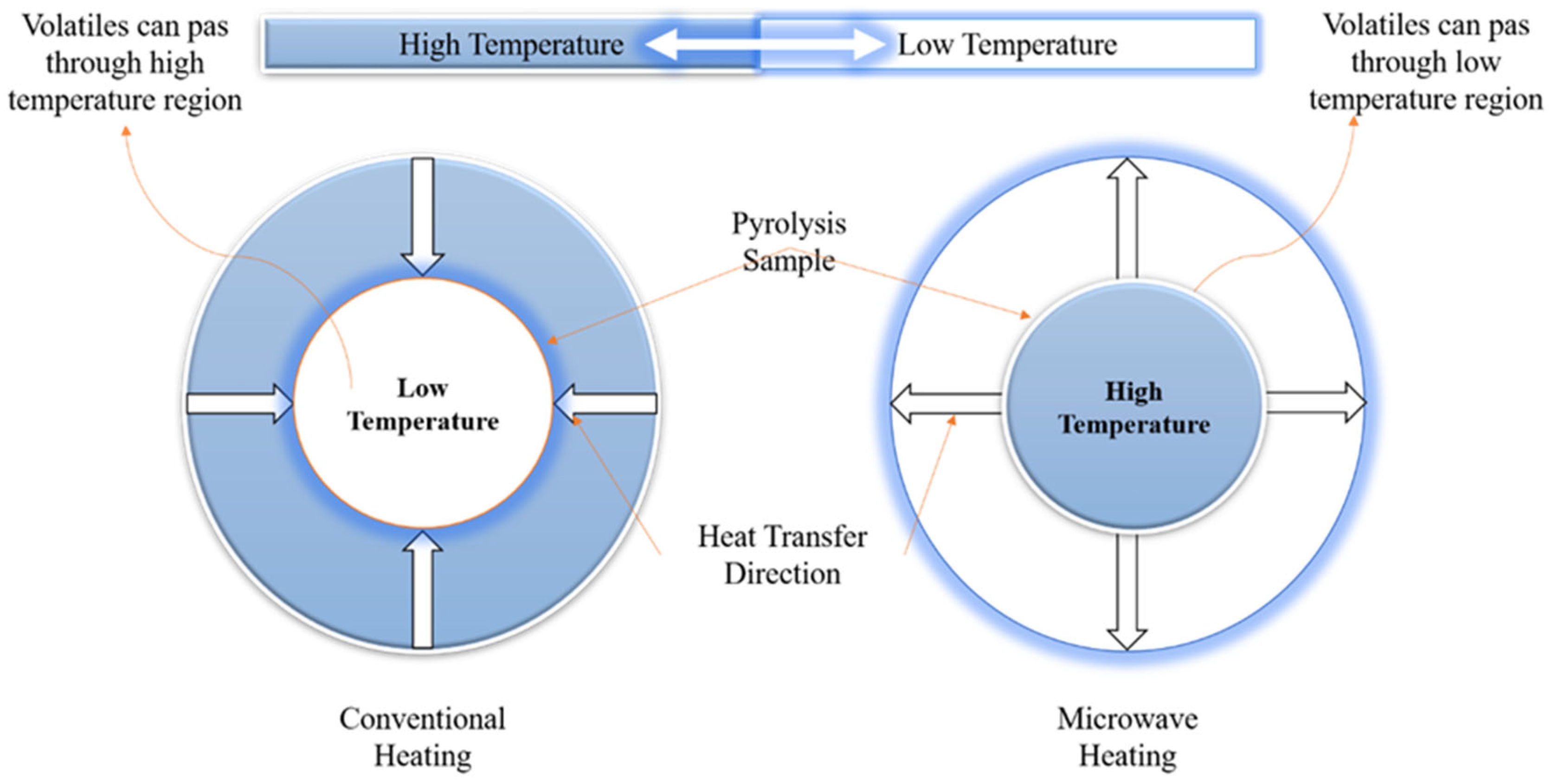
Figure 2.
Comparison of microwave pyrolysis with the conventional pyrolysis of biomass. Recreated with permission from Ref. [62]. Copyright © 2003 Elsevier B.V.
References
- Hatfield-Dodds, S.; Schandl, H.; Newth, D.; Obersteiner, M.; Cai, Y.; Baynes, T.; West, J.; Havlik, P. Assessing global resource use and greenhouse emissions to 2050, with ambitious resource efficiency and climate mitigation policies. J. Clean. Prod. 2017, 144, 403–414.
- Lee, H.W.; Kim, Y.M.; Kim, S.; Ryu, C.; Park, S.H.; Park, Y.K. Review of the use of activated biochar for energy and environmental applications. Carbon Lett. 2018, 26, 1–10.
- Merlet, C.; Rotenberg, B.; Madden, P.A.; Taberna, P.-L.; Simon, P.; Gogotsi, Y.; Salanne, M. On the molecular origin of supercapacitance in nanoporous carbon electrodes. Nat. Mater. 2012, 11, 306–310.
- Qian, K.; Kumar, A.; Zhang, H.; Bellmer, D.; Huhnke, R. Recent advances in utilization of biochar. Renew. Sustain. Energy Rev. 2015, 42, 1055–1064.
- Melikoglu, M. Pumped hydroelectric energy storage: Analysing global development and assessing potential applications in Turkey based on Vision 2023 hydroelectricity wind and solar energy targets. Renew. Sustain. Energy Rev. 2017, 72, 146–153.
- Chen, Z.; Mo, F.; Wang, T.; Yang, Q.; Huang, Z.; Wang, D.; Liang, G.; Chen, A.; Li, Q.; Guo, Y.; et al. Zinc/selenium conversion battery: A system highly compatible with both organic and aqueous electrolytes. Energy Environ. Sci. 2021, 14, 2441–2450.
- Bi, Z.; Kong, Q.; Cao, Y.; Sun, G.; Su, F.; Wei, X.; Li, X.; Ahmad, A.; Xie, L.; Chen, C.-M. Biomass-derived porous carbon materials with different dimensions for supercapacitor electrodes: A review. J. Mater. Chem. A 2019, 7, 16028–16045.
- Zhang, H.; He, X.; Wei, F.; Dong, S.; Xiao, N.; Qiu, J. Moss-covered rock-like hybrid porous carbons with enhanced electrochemical properties. ACS Sustain. Chem. Eng. 2020, 8, 3065–3071.
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828.
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. In Nanoscience and Technology: A Collection of Reviews from Nature Journals; World Scientific: Singapore, 2010; pp. 320–329.
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531.
- Matsagar, B.M.; Wu, K.C.W. Agricultural waste-derived biochar for environmental management. In Biochar in Agriculture for Achieving Sustainable Development Goals; Elsevier: Amsterdam, The Netherlands, 2022; pp. 3–13.
- Sun, L.; Tian, C.; Li, M.; Meng, X.; Wang, L.; Wang, R.; Yin, J.; Fu, H. From coconut shell to porous graphene-like nanosheets for high-power supercapacitors. J. Mater. Chem. A 2013, 1, 6462–6470.
- Saikia, B.K.; Benoy, S.M.; Bora, M.; Tamuly, J.; Pandey, M.; Bhattacharya, D. A brief review on supercapacitor energy storage devices and utilization of natural carbon resources as their electrode materials. Fuel 2020, 282, 118796.
- Yakaboylu, G.A.; Jiang, C.; Yumak, T.; Zondlo, J.W.; Wang, J.; Sabolsky, E.M. Engineered hierarchical porous carbons for supercapacitor applications through chemical pretreatment and activation of biomass precursors. Renew. Energy 2020, 163, 276–287.
- Ye, Y.-Y.; Qian, T.-T.; Jiang, H. Co loaded N-doped Biochar as a High Performance of Oxygen Reduction Reaction Electrocatalyst by Combined Pyrolysis of Biomass. Ind. Eng. Chem. Res. 2020, 59, 15614–15623.
- Garg, R.; Elmas, S.; Nann, T.; Andersson, M.R. Deposition methods of graphene as electrode material for organic solar cells. Adv. Energy Mater. 2016, 7, 1601393.
- Deng, J.; Li, M.; Wang, Y. Biomass-derived carbon: Synthesis and applications in energy storage and conversion. Green Chem. 2016, 18, 4824–4854.
- Deng, J.; Xiong, T.; Wang, H.; Zheng, A.; Wang, Y. Effects of cellulose, hemicellulose, and lignin on the structure and morphology of porous carbons. ACS Sustain. Chem. Eng. 2016, 4, 3750–3756.
- Chu, G.; Zhao, J.; Huang, Y.; Zhou, D.; Liu, Y.; Wu, M.; Peng, H.; Zhao, Q.; Pan, B.; Steinberg, C.E. Phosphoric acid pretreatment enhances the specific surface areas of biochars by generation of micropores. Environ. Pollut. 2018, 240, 1–9.
- Sharma, R.; Wooten, J.; Baliga, V.; Hajaligol, M. Characterization of chars from biomass-derived materials: Pectin chars. Fuel 2001, 80, 1825–1836.
- Matsagar, B.M.; Yang, R.-X.; Dutta, S.; Ok, Y.S.; Wu, K.C.-W. Recent progress in the development of biomass-derived nitrogen-doped porous carbon. J. Mater. Chem. A 2020, 9, 3703–3728.
- Sajjadi, B.; Chen, W.-Y.; Egiebor, N.O. A comprehensive review on physical activation of biochar for energy and environmental applications. Rev. Chem. Eng. 2018, 35, 735–776.
- Sajjadi, B.; Zubatiuk, T.; Leszczynska, D.; Leszczynski, J.; Chen, W.Y. Chemical activation of biochar for energy and environmental applications: A comprehensive review. Rev. Chem. Eng. 2018, 35, 777–815.
- Huang, W.-H.; Lee, D.-J.; Huang, C. Modification on biochars for applications: A research update. Bioresour. Technol. 2020, 319, 124100.
- Shen, F.; Xiong, X.; Fu, J.; Yang, J.; Qiu, M.; Qi, X.; Tsang, D.C. Recent advances in mechanochemical production of chemicals and carbon materials from sustainable biomass resources. Renew. Sustain. Energy Rev. 2020, 130, 109944.
- Gaudino, E.C.; Cravotto, G.; Manzoli, M.; Tabasso, S. Sono- and mechanochemical technologies in the catalytic conversion of biomass. Chem. Soc. Rev. 2020, 50, 1785–1812.
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction: Practical Design and Theory; Academic Press: Cambridge, MA, USA, 2018.
- Xiu, S.; Shahbazi, A.; Li, R. Characterization, modification and application of biochar for energy storage and catalysis: A review. Trends Renew. Energy 2017, 3, 86–101.
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378.
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Bimetallic catalysts for upgrading of biomass to fuels and chemicals. Chem. Soc. Rev. 2012, 41, 8075–8098.
- Leng, L.; Xiong, Q.; Yang, L.; Li, H.; Zhou, Y.; Zhang, W.; Jiang, S.; Li, H.; Huang, H. An overview on engineering the surface area and porosity of biochar. Sci. Total Environ. 2020, 763, 144204.
- Maggi, R.; Delmon, B. Comparison between ‘slow’and ‘flash’pyrolysis oils from biomass. Fuel 1994, 73, 671–677.
- Román, S.; Valente Nabais, J.M.; Ledesma, B.; González, J.F.; Laginhas, C.; Titirici, M.M. Production of low-cost adsorbents with tunable surface chemistry by conjunction of hydrothermal carbonization and activation processes. Microporous Mesoporous Mater. 2013, 165, 127–133.
- Panwar, N.L.; Pawar, A. Influence of activation conditions on the physicochemical properties of activated biochar: A review. Biomass Convers. Biorefinery 2020, 12, 925–947.
- Demirbaş, A. Mechanisms of liquefaction and pyrolysis reactions of biomass. Energy Convers. Manag. 2000, 41, 633–646.
- Goldstein, I.S. Organic Chemicals from Biomass; CRC Press: Boca Raton, FL, USA, 2018.
- Khan, S.R.; Zeeshan, M.; Masood, A. Enhancement of hydrocarbons production through co-pyrolysis of acid-treated biomass and waste tire in a fixed bed reactor. Waste Manag. 2020, 106, 21–31.
- Khan, S.R.; Zeeshan, M. Catalytic potential of low-cost natural zeolite and influence of various pretreatments of biomass on pyro-oil up-gradation during co-pyrolysis with scrap rubber tires. Energy 2021, 238, 121820.
- Ma, Z.; Yang, Y.; Wu, Y.; Xu, J.; Peng, H.; Liu, X.; Zhang, W.; Wang, S. In-depth comparison of the physicochemical characteristics of bio-char derived from biomass pseudo components: Hemicellulose, cellulose, and lignin. J. Anal. Appl. Pyrolysis 2019, 140, 195–204.
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788.
- Sun, Y.; Yu, I.K.; Tsang, D.C.; Fan, J.; Clark, J.H.; Luo, G.; Zhang, S.; Khan, E.; Graham, N.J. Tailored design of graphitic biochar for high-efficiency and chemical-free microwave-assisted removal of refractory organic contaminants. Chem. Eng. J. 2020, 398, 125505.
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Bio Technol. 2020, 19, 191–215.
- Sun, K.; Kang, M.; Zhang, Z.; Jin, J.; Wang, Z.; Pan, Z.; Xu, D.; Wu, F.; Xing, B. Impact of deashing treatment on biochar structural properties and potential sorption mechanisms of phenanthrene. Environ. Sci. Technol. 2013, 47, 11473–11481.
- Antal, M.J.; Grønli, M. The art, science, and technology of charcoal production. Ind. Eng. Chem. Res. 2003, 42, 1619–1640.
- Kumar, J.V.; Pratt, B.C. Compositional analysis of some renewable biofuels. Am. Lab. 1996, 28, 15–20.
- Irfan, M.; Chen, Q.; Yue, Y.; Pang, R.; Lin, Q.; Zhao, X.; Chen, H. Co-production of biochar, bio-oil and syngas from halophyte grass (Achnatherum splendens L.) under three different pyrolysis temperatures. Bioresour. Technol. 2016, 211, 457–463.
- Pallarés, J.; González-Cencerrado, A.; Arauzo, I. Production and characterization of activated carbon from barley straw by physical activation with carbon dioxide and steam. Biomass Bioenergy 2018, 115, 64–73.
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation; Routledge: Abingdon, UK, 2015.
- Leng, L.; Huang, H. An overview of the effect of pyrolysis process parameters on biochar stability. Bioresour. Technol. 2018, 270, 627–642.
- Zhao, B.; O’Connor, D.; Zhang, J.; Peng, T.; Shen, Z.; Tsang, D.C.W.; Hou, D. Effect of pyrolysis temperature, heating rate, and residence time on rapeseed stem derived biochar. J. Clean. Prod. 2018, 174, 977–987.
- Chen, D.; Li, Y.; Cen, K.; Luo, M.; Li, H.; Lu, B. Pyrolysis polygeneration of poplar wood: Effect of heating rate and pyrolysis temperature. Bioresour. Technol. 2016, 218, 780–788.
- Bouchelta, C.; Medjram, M.S.; Zoubida, M.; Chekkat, F.A.; Ramdane, N.; Bellat, J.-P. Effects of pyrolysis conditions on the porous structure development of date pits activated carbon. J. Anal. Appl. Pyrolysis 2012, 94, 215–222.
- Liu, R.; Liu, G.; Yousaf, B.; Abbas, Q. Operating conditions-induced changes in product yield and characteristics during thermal-conversion of peanut shell to biochar in relation to economic analysis. J. Clean. Prod. 2018, 193, 479–490.
- Sakhiya, A.K.; Anand, A.; Kaushal, P. Production, activation, and applications of biochar in recent times. Biochar 2020, 2, 253–285.
- Sahoo, S.S.; Vijay, V.K.; Chandra, R.; Kumar, H. Production and characterization of biochar produced from slow pyrolysis of pigeon pea stalk and bamboo. Clean. Eng. Technol. 2021, 3, 100101.
- Sun, Y.; Gao, B.; Yao, Y.; Fang, J.; Zhang, M.; Zhou, Y.; Chen, H.; Yang, L. Effects of feedstock type, production method, and pyrolysis temperature on biochar and hydrochar properties. Chem. Eng. J. 2014, 240, 574–578.
- Malekshahian, M.; Hill, J.M. Effect of pyrolysis and CO2 gasification pressure on the surface area and pore size distribution of petroleum coke. Energy Fuels 2011, 25, 5250–5256.
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140.
- Salema, A.A.; Afzal, M.T. Numerical simulation of heating behaviour in biomass bed and pellets under multimode microwave system. Int. J. Therm. Sci. 2015, 91, 12–24.
- Namazi, A.B.; Allen, D.; Jia, C.Q. Probing microwave heating of lignocellulosic biomasses. J. Anal. Appl. Pyrolysis 2015, 112, 121–128.
- Zhang, T.; Walawender, W.P.; Fan, L.; Fan, M.; Daugaard, D.; Brown, R. Preparation of activated carbon from forest and agricultural residues through CO2 activation. Chem. Eng. J. 2004, 105, 53–59.
- Lu, H.; Zhao, X.S. Biomass-derived carbon electrode materials for supercapacitors. Sustain. Energy Fuels 2017, 1, 1265–1281.
- Cheng, B.-H.; Zeng, R.J.; Jiang, H. Recent developments of post-modification of biochar for electrochemical energy storage. Bioresour. Technol. 2017, 246, 224–233.
- Liu, W.-J.; Jiang, H.; Yu, H.-Q. Development of biochar-based functional materials: Toward a sustainable platform carbon material. Chem. Rev. 2015, 115, 12251–12285.
More
