Rickettsia felis in an obligate intracellular Gram negative bacterium and the causative agent of flea-borne spotted fever (FBSF). Rickettsia felis requires a vertebrate and invertebrate host to survive and reproduce. The cat flea (Ctenocephalides felis) is considered as the primary vector and the reservoir host of this pathogen.
- Rickettsia felis
- rickettsiosis
- zoonosis
1. Introduction
Rickettsia felis requires a vertebrate and invertebrate host to survive and reproduce. The cat flea (Ctenocephalides felis) is considered as the primary vector and the reservoir host of this pathogen [2,5]. Rickettsia felis has been also identified in various flea species and there is a growing evidence of detection in other arthropods: ticks, mites, lice and mosquitoes. Similarly, the host range of R. felis is increasing; reports on infected humans, domestic and wild animals are coming from all over the world. However, the competency of the different arthropods and hosts as vectors and reservoirs, respectively, is yet to be demonstrated [5].
2. Vectors and Hosts of R. felis in Europe
2.1. Vectors
During 2017–2022, a total of 11 European countries reported the occurrence of R. felis in several vector species (Figure 1). The vectors found to be infected included flea, tick and mite species; the dominant flea and tick species were C. felis and I. ricinus, respectively. The baseline characteristics of the studies on vectors which were included in Table 1.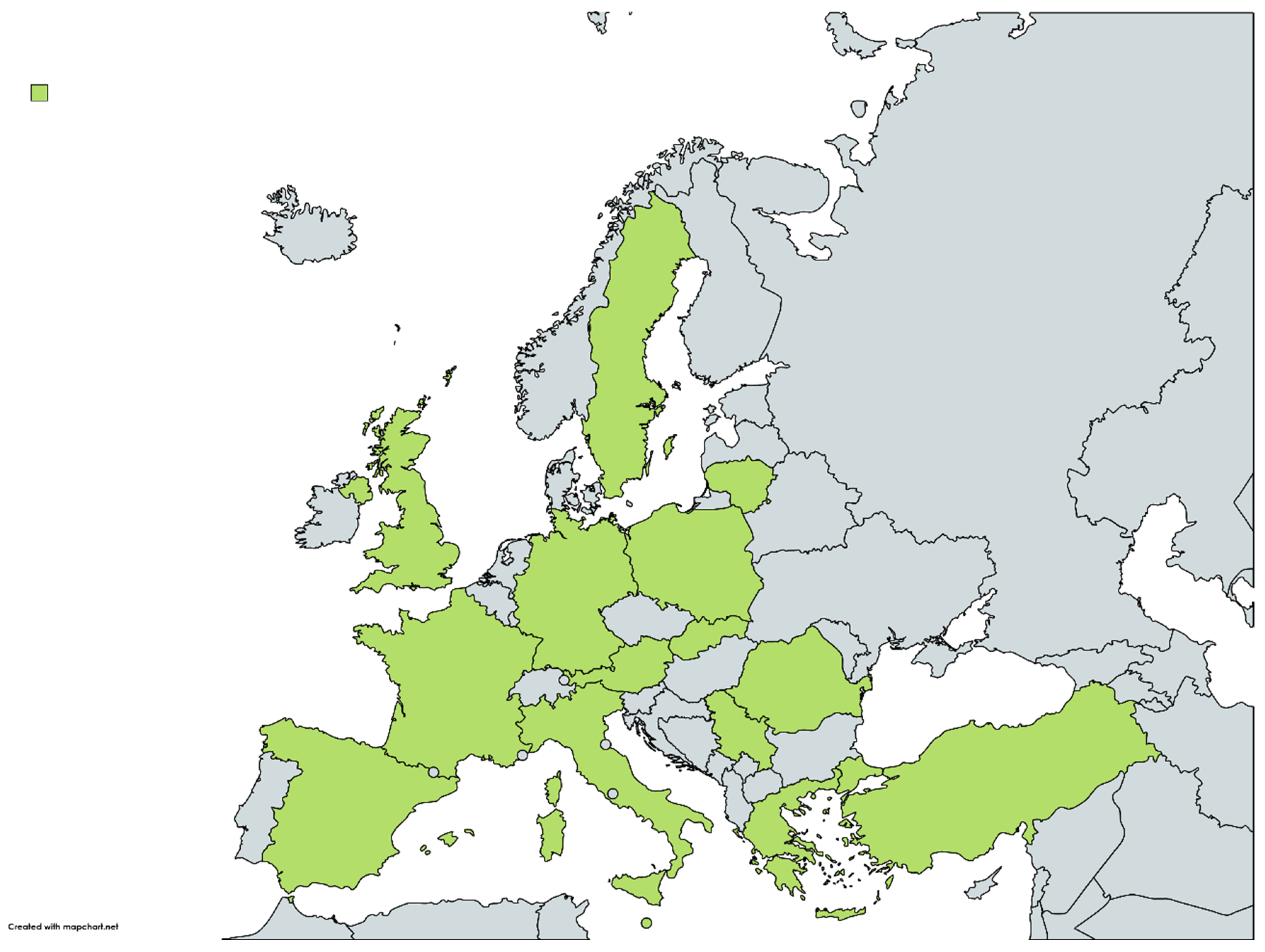
| Countries | Study Period | Vectors | Prevalence in Vector | Vector Hosts | Reference |
|---|
| Countries | Study Period | Host | Prevalence in Host | Reference | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Austria | 2016 | C. felis | Not defined (1/105) | Cats | [ | 14 | ] | |||||||||||||
| Germany | 2008 | Human | 2.7% (15/559) * | [ | 34 | ] | ||||||||||||||
| France | 2014–2017 | I. ricinus | 0.1% (1/998) | Environment | [ | 25 | ] | |||||||||||||
| Germany | 2010–2014 | Wild mammals ( | A. amphibious | , | A. flavicollis | , | A. sylvaticus | ) | Not defined | [ | 39 | ] | France | 2017 | I. ricinus | 7% ** | Environment | [ | 26 | ] |
| Germany | 2012–2014 | Small mammals ( | A. flavicollis | ) | Not defined | [ | Greece | 2013 | C. felis | 13% (3/23) | Cats | [ | 16 | ] | ||||||
| 27 | ] | |||||||||||||||||||
| Greece | 2013 | Human | 3.5% (8/223) * | [ | 16 | ] | Greece | 2016–2017 | C. felis | , | C. canis | , | P. irritans | 14% (14/100) * | Dogs and Cats | [ | 17 | ] | ||
| Italy | 2010–2016 | Cats | 8.04% (23/286) * | [ | 36 | ] | Italy | 2013 | Rh. turanicus | 2.9% (1/34) * | Sheep | |||||||||
| Italy | 2018–2021 | [ | Cats | 28 | ] | |||||||||||||||
| 17.89% (17/95) * | [ | 37 | ] | Italy | 2014–2016 | I. hexagonus | Not defined | Hedgehog and fox | ||||||||||||
| Malta | 2017 | [ | Cats | 29 | ] | |||||||||||||||
| 0% | [ | 20 | ] | Lithuania | 2013–2014 | H. microti | , | L. agilis | , | Ct. agyrtes | , | H. talpae | Not defined | Rodents | [ | 18 | ] | |||
| Poland | 2014 | Small mammals ( | A. flavicollis | ) | Not defined | [ | 40 | ] | Malta | 2017 | C. felis | 39.47% (15/38) | Cats | |||||||
| Serbia | 2019 | [ | Human | 19 | ] | |||||||||||||||
| 3% (1/30) | [ | 31 | ] | Malta | 2017 | C. felis | 96.42% (54/56) * | Serbia | 2020 | Human | Not defined (1/85)Cats | [ | 32 | ][ | 20 | ] | ||||
| Romania | 2018 | I. ricinus | Not defined (1/222) | Rodents, birds, hedgehogs | [ | 30 | ] | |||||||||||||
| Slovakia | Serbia | 2019 | I. ricinus | 3% (1/31) | Humans | [ | 31 | ] | ||||||||||||
| Serbia | 2020 | Ticks | 4.3% | Humans | [ | 32 | ] | |||||||||||||
| Slovakia | 2012–2014 | N. fasciatus | , | Ct. assimilis | Not defined | Rodents | [ | 22 | ] | |||||||||||
| Slovakia | 2014–2016 | Ct. solutus | Not defined | Small mammals ( | A. agrarius | ) | [ | 21 | ] | |||||||||||
| Spain | 2011–2018 | C. felis | 28.3% (15/53) | Dogs | [ | 15 | ] | |||||||||||||
| A. erinacei | 33.3% (6/18) | Hedgehogs | ||||||||||||||||||
| Ct. b. boisseauorum | 1.6% (1/60) | Rodents ( | A. terrestris | ) | ||||||||||||||||
| Spain | 2015–2017 | I. ricinus | 0.46% (1/219) | Environment | [ | 33 | ] | |||||||||||||
| Spain | 2019–2020 | C. felis | 29.6% (38/128) | Dogs and Cats | [ | 23 | ] | |||||||||||||
| UK | 2018 | C. felis | , | C. canis | 5.7% (27/470) * | Dogs and Cats | [ | 24 | ] |
2.2. Hosts
During 2017–2022, a total of nine European countries reported the occurrence of R. felis in different hosts (Figure 1). The hosts found to be infected by R. felis by molecular methods or exposed to R. felis by serology were humans, cats and small mammals. The baseline characteristics of the studies on hosts which were included in Table 2.| 2014–2015 | ||||||
| Small mammals | ||||||
| ( | ||||||
| A. flavicollis | ||||||
| ) | ||||||
| 1.1% (3/27) | ||||||
| [ | 41 | ] | ||||
| Sweden | 2015 | Human | Not defined * | [ | 35 | ] |
| Turkey | 2017–2021 | Cats | 26.3% (44/167) | [ | 38 | ] |
