Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Angela santoro and Version 2 by Rita Xu.
Cervical cancer is one of the most common cancers worldwide, ranking as fourth for both incidence and mortality among all gynecological malignancies. Squamous cell carcinoma (SCC) is the most frequent histotype, followed by adenocarcinoma (AC), which accounts for approximately 10–25% of cervical tumors.
- cervical cancer
- prognosis
- grading
1. Introduction
Despite the majority of cases, especially in developing countries, being diagnosed at an advanced stage, an increasing percentage of tumors are diagnosed at an early stage [1][2][1,2]. For early-stage disease, several pathological parameters, including tumor size, histotype, depth of stromal invasion, lympho-vascular space invasion (LVSI) and lymph node status have been proposed as prognostic predictors, capable of stratifying patients into different risk groups [3][4][5][6][3,4,5,6].
However, given the high mortality and recurrence rate of cervical cancer, the abovementioned prognostic factors are still of limited value and provide suboptimal prognostic stratification for recurrence [1][3][1,3]. Therefore, novel parameters, able to provide additional prognostic information, are needed in order to allow a better clinical stratification of cervical cancer patients.
Actually, the histopathology report for cervical carcinoma should include all relevant information required for diagnosis, staging, prognosis and patient management. According to the most recent recommendations from College of American Pathologists (CAP) and the International Collaboration on Cancer Reporting (ICCR) [4], the following essential items should be included in the pathology report:
- -
-
macroscopic tumor site
- -
-
tumor dimensions (measurements of horizontal extent and depth of invasion or tumor thickness)
- -
-
maximum and minimum length of vaginal cuff and parametria in two dimensions
- -
-
histological tumor type and tumor grade
- -
-
coexistent pathology (squamous intraepithelial lesion, adenocarcinoma in situ, stratified mucin-producing intraepithelial lesion)
- -
-
LVSI
- -
-
minimum distance of uninvolved cervical stroma
- -
-
extent of invasion (vaginal, uterine corpus, parametrial, adnexal, bladder, rectum involvement)
- -
-
margin status (for invasive tumors and for precursor lesions)
- -
-
lymph node status: sentinel lymph node status, total number of nodes retrieved, number of positive lymph nodes
- -
-
pathologically confirmed distant metastases.
Moreover, the following desired/recommended items should also be included in the pathology report:
- -
-
HPV dependent and independent status
- -
-
Silva pattern of invasion
- -
-
Ancillary studies (p16 immunohistochemistry; in-situ hybridization for HPV).
2. Prognostic Factors in Primary Neoplasm
The most relevant prognostic factors in primary tumors are summarized in Table 1 and Table 2.Table 1. Prognostic factors related to primary tumors: squamous cell carcinoma.
| Squamous Cell Carcinoma | ||||
|---|---|---|---|---|
| Established | Prognostic Factor | Novel Prognostic Factor | Uncertain Prognostic Utility | |
Table 2. Prognostic factors related to primary tumors: adenocarcinoma.
| Adenocarcinoma | ||||
|---|---|---|---|---|
| Established | Prognostic Factor | Novel Prognostic Factor | Uncertain Prognostic Utility | |
| HPV status | Tumor-budding/Cell nest size | Grading | ||
| Depth of stomal invasion LVSI Parametrial extension | ||||
| HPV status | Tumor-budding/Cell nest size | Grading Neuroendocrine differentiation Horizontal extension |
||
| Margin status | Tumor-free distance (TFD) Perineural Invasion (PNI) |
Horizontal extension | ||
| Silva pattern of invasion | TILS | |||
| Depth of stomal invasion LVSI | |
| Tumor-free distance (TFD) | |
| Parametrial extension Margin status |
Perineural Invasion (PNI) |
| TILS | |
| Special histologic types (gastric-type, clear cell, mesonephric, micropapillary, signet ring, invasive stratified mucinous carcinoma) |
2.1. HPV Status
HPV infection represents the main pathogenetic event leading to cervical cancer development. The key step in the pathogenesis of cervical carcinomas is the integration of the HPV genome into the host chromosome, followed by the inactivation of viral E1 and E2 regions and upregulation of oncogenes E6 and E7 [7]. In detail, E6 oncoprotein degrades p53, inhibiting apoptosis while E7 protein stimulates cell proliferation by suppressing RB1 [7]. Despite the vast majority of cervical epithelial tumors being related to HPV infection, it is now recognized that a proportion of these tumors, mainly represented by adenocarcinomas, are not associated with HPV infection and carry more aggressive clinical behavior than HPV-related carcinomas [2][4][2,4]. In this regard, the 2020 WHO Classification of female genital tumors introduced a novel classification for cervical epithelial tumors based on the presence or absence of HPV infection [2]. In detail, adenocarcinomas are now categorized into HPV-associated (HPVA) and HPV-independent tumors (HPVI). This latter group includes the following histotypes: gastric-type, clear cell, mesonephric and endometrioid carcinoma [2][4][2,4]. On the other hand, the 2020 WHO Classification categorizes squamous epithelial tumors into HPV-associated and HPV-independent categories [2]. HPV-independent squamous cell carcinoma is exceedingly rare and shows a higher rate of lymph node metastases, with a consequent reduced disease-free and overall survival compared with HPV-associated carcinoma [8]. However, currently there are not yet differences in treatment strategies between HPV-associated and HPV-independent tumors. The same distinction (HPV-dependent/HPV-independent) is applied for premalignant precursor lesions, squamous and glandular in type:- -
-
HPV-dependent SILs (squamous intraepithelial lesions): high grade and low grade, respectively corresponding to CIN1 and CIN2-3 dysplasia
- -
-
HPV-dependent AIS (adenocarcinoma in-situ) and its SMILE variant (stratified mucin producing intraepithelial lesion)
- -
-
HPV-independent AIS: gastric-type AIS and ALEGH (atypical lobular endocervical glandular hyperplasia)
2.2. Grading of Cervical Cancer
To date, there is no widespread consensus regarding the prognostic significance of tumor grade, and no validated grading systems are currently available for cervical cancer [4][10][11][12][13][14][15][16][17][18][19][4,10,11,12,13,14,15,16,17,18,19]. Although tumor grading is considered a recommended (not required) pathological feature, in the recent recommendations of the European Society of Gynaecological Oncology (ESGO), the European Society for Radiotherapy and Oncology (ESTRO) and the European Society of Pathology (ESP), it is not taken into account in clinical management of the cervical cancer patients to assess the need for adjuvant therapy following surgery [10][11][12][13][14][15][16][17][18][19][10,11,12,13,14,15,16,17,18,19]. Similarly, the recent ICCR data set for the reporting of cervical cancers and the “Sedlis Criteria” do not take into consideration grading for adjuvant treatment algorithms [4].2.2.1. Squamous Cell Carcinoma
Several grading systems are currently applied to grade cervical squamous cell carcinoma [12][13][14][15][16][17][18][12,13,14,15,16,17,18]; these include:- -
-
the Broder’s system, based on the degree of keratinization, cytological atypia and mitotic activity;
- -
-
the grading of invasive tumor front or the pattern-type of invasion (pushing versus infiltrative);
- -
-
the typology of neoplastic cells and the presence/absence of keratinization (large-cell keratinizing, large-cell non-keratinizing, and small-cell non-keratinizing categories);
- -
-
the WHO proposal that considers the degree of keratinization, nuclear pleomorphism, size of nucleoli and mitotic index.
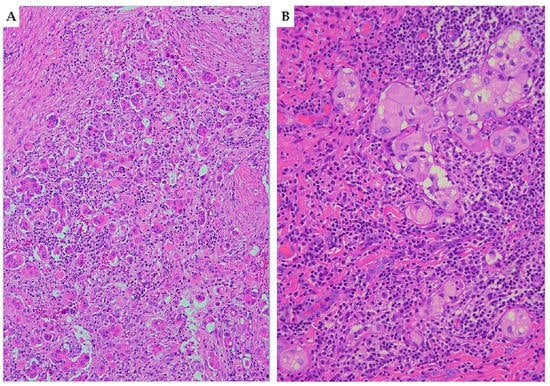 Figure 1. Haematoxylin and eosin (H&E) stained sections ((A) 10×; (B) 20×) illustrating tumor-budding and cell nest size in squamous cell carcinoma of the cervix. (A) Cervical squamous cell carcinoma with high tumor-budding activity, characterized by numerous small tumor clusters of <5 cells present at the infiltrating edge of the tumor (×10). (B) Small-sized cell nests: cervical cancer showing cell nests consisting of 2–4 tumor cells with nested architecture (×20).
Figure 1. Haematoxylin and eosin (H&E) stained sections ((A) 10×; (B) 20×) illustrating tumor-budding and cell nest size in squamous cell carcinoma of the cervix. (A) Cervical squamous cell carcinoma with high tumor-budding activity, characterized by numerous small tumor clusters of <5 cells present at the infiltrating edge of the tumor (×10). (B) Small-sized cell nests: cervical cancer showing cell nests consisting of 2–4 tumor cells with nested architecture (×20).2.2.2. Adenocarcinoma
Several studies suggest a grading system for HPVA adenocarcinomas based on a combination of architectural and nuclear features and similar to the FIGO grading system applied for uterine endometrioid carcinomas [27][28][27,28]. The most commonly used cut-offs for solid architecture set at ≤10% (grade 1), 11% to 50% (grade 2), and >50% (grade 3) has been recommended, due to its good prognostic significance. Tumors can be upgraded in the presence of marked nuclear atypia in the majority of cells (>50%) [7]. Moreover, a clearly defined subset of endocervical adenocarcinomas should be considered intrinsically high-grade regardless of the morphology. Most of these represent HPVI adenocarcinomas (gastric type adenocarcinoma, clear cell carcinoma and mesonephric adenocarcinoma). Among the cervical HPVA adenocarcinomas, the following variant should be not graded because it is considered automatically high-grade: micropapillary carcinomas, mucinous adenocarcinomas and neuroendocrine carcinomas (also in the mixed forms, irrespective of the percentage of the neuroendocrine component) [29][30][31][29,30,31]. A recent paper by Shi et al. demonstrated the reliability of the grading scheme based on tumoral budding and small nest size also in endocervical adenocarcinomas where it seems to outperform the conventional Federation of Gynecology and Obstetrics (FIGO) grading and Silva pattern classification [32]. Therefore, if further studies on larger cohorts will show similar results, the novel grading system could be included in the pathology reports as an additional tool to guide the therapeutic management of cervical cancer patients.2.3. Silva Pattern of Invasion for HPV-Associated Adenocarcinomas
Recently, the Silva Pattern Classification has been shown to correlate with the risk of lymph node metastasis and patient survival [4][33][4,33]. The Silva classification can only be applied to HPVA cervical adenocarcinoma and sub-classifies tumors into three patterns (A, B, C) based on the presence and degree of destructive stromal invasion, LVSI and grade of cytologic atypia [33]. In detail, Pattern A tumors are composed of well-formed glands without evidence of destructive stromal invasion, single cells, solid growth, high grade cytology or LVSI. Pattern B tumors show limited destructive invasion with individual cells or clusters of tumor cells not exceeding 5 mm in maximum diameter. Pattern C tumors are characterized by diffuse destructive invasion associated with desmoplastic reaction. The current literature evidence, mainly based on retrospective studies, suggests that Pattern A tumors do not develop lymph node metastases and carry a very limited risk of recurrence; therefore, they can be suitable for conservative treatment without lymph node dissection [2]. The risk of lymph node metastases is very low for Pattern B adenocarcinomas, which may benefit from SLN mapping, especially if LVSI is present. Finally, Pattern C tumors have a more significant risk of nodal metastases and tumor recurrences; therefore, standard surgical treatment, including lymph node dissection, is more appropriate for these latter patients [2].2.4. Lympho-Vascular Space Invasion (LVSI)
LVSI assessment is a required item in the pathology report of cervical cancer since it is one of the criteria used to select patients suitable for surgical radicality and adjuvant treatment [11]. Several studies have investigated the prognostic role of LVSI and its association with nodal and distant metastases and patient survival [11][34][35][36][37][38][39][11,34,35,36,37,38,39]. However, results are extremely heterogeneous since some studies have shown the negative prognostic role of LVSI while other studies failed to demonstrate statistically significant results [11][37][38][39][11,37,38,39]. This discrepancy across studies may be explained by the qualitative method utilized to assess LVSI: present or absent. In this regard, literature data in endometrial cancer patients demonstrated that a semi-quantitative evaluation may better stratify patient prognosis [36][37][38][39][36,37,38,39]. In detail, according to the “three-tiered approach” for endometrial cancer, LVSI has been classified as follows: (i) Absent: No LVSI; (ii) Focal: single focus of LVSI around the tumor; (iii) Diffuse: more than 1 focus around the tumor [40][41][42][43][40,41,42,43]. With this approach, a diffuse pattern of LVSI has been demonstrated as an independent prognostic factor for nodal metastases, recurrence and decreased survival; on the other hand, endometrial cancer patients with focal LVSI showed a significantly better outcome [40][41][42][43][40,41,42,43]. Regarding LVSI in cervical cancer, a recent study by Ronsini et al. demonstrated, for the first time, that a semi-quantitative evaluation of LVSI in early-stage cervical cancer patients could provide a more accurate survival stratification [43]. In detail, different clinico-pathological features and survival outcomes were observed in patients with absent, focal and diffuse LVSI, respectively. Moreover, diffuse LVSI was associated with increased risk of nodal metastases, parametrial involvement and positive surgical margins [43]. Literature data also showed that only LVSI outside the tumors border, so called satellite LVSI rather than intratumor LVSI, has a significant prognostic value in cervical cancer [44]. If future studies on large series will support these findings, a semi-quantitative evaluation of LVSI could be recommended in the pathology reports of cervical cancer patients in order to optimize the diagnostic and therapeutic process.2.5. Perineural Invasion (PNI)
PNI, according to the Liebig criteria, is defined as the presence of tumor cells along the nerve circumference or invading any of the three layers of the nerve sheath (epineurium, perineurium and endoneurium) (Figure 2) [45]. PNI is frequently detected in several malignancies, including head and neck squamous cell carcinoma, colorectal adenocarcinoma, prostate cancer, cholangiocarcinoma and pancreatic cancer [45][46][47][48][49][50][45,46,47,48,49,50]. In cervical cancer, the reported incidence of PNI ranges from 7.0 to 35.1%; moreover, PNI is frequently detected in combination with other risk factors, such as LVSI, deep cervical invasion, large tumor size, tumor extension to the uterus, positive surgical margins, parametrial invasion and pelvic lymph node metastases [50][51][52][53][50,51,52,53]. Therefore, patients with PNI are more likely to receive adjuvant radiotherapy or concurrent chemo-radiation after surgery. However, the real prognostic impact of PNI in cervical cancer is poorly understood and is still a matter of debate. In detail, some studies demonstrated the role of PNI as independent prognostic factor for OS; other studies showed a significant correlation of PNI with DFS and OS at univariate but not at multivariate analysis, whereas other authors failed to demonstrate any prognostic role of PNI [50][51][52][53][50,51,52,53]. Despite its limited prognostic role, it is well known that PNI is frequently related to other poor prognostic factors, such as LVSI, deep stromal invasion, large tumor size and parametrium invasion; therefore, its real impact on prognosis needs to be better elucidated [50][51][52][53][50,51,52,53]. According to literature data, PNI could be considered as an intermediate-risk factor for cervical cancer patients that may aid in the selection of the more appropriate therapeutic approach [50][51][52][53][50,51,52,53].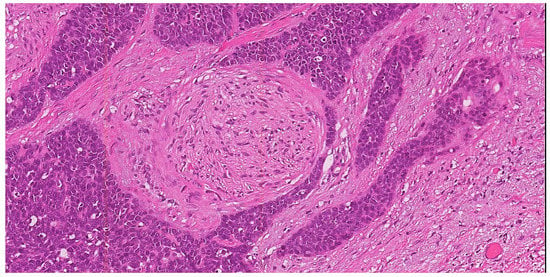 Figure 2. H&E stained section (10×) illustrating perineural invasion. In this example of squamous cell carcinoma of the uterine cervix with basaloid morphology, a small round nerve structure (center of the field) is surrounded by the neoplastic proliferation (×10).
Figure 2. H&E stained section (10×) illustrating perineural invasion. In this example of squamous cell carcinoma of the uterine cervix with basaloid morphology, a small round nerve structure (center of the field) is surrounded by the neoplastic proliferation (×10).2.6. Depth of Stromal Invasion (DOI)
Depth of stromal invasion (DOI) represents an essential tool to be included in the pathology report, not only for staging purposes but also for its potential role as prognostic factor in cervical cancer [5]. According to Sedlis criteria, DOI is expressed as inner third, middle third and outer third of cervical wall thickness infiltration [54]. Several studies showed that DOI represents an independent prognostic factor for OS and DFS and is strictly related to local recurrences. Moreover, a significant difference of prognosis has been demonstrated between tumors with full-thickness invasion and tumors reaching the cervical–parametrial transition zone [54][55][54,55]. According to recent studies, DOI may represent a reliable method to categorize the pathological tumor response in cervical cancer after neoadjuvant therapy [5][55][5,55]. In detail, a recent meta-analysis evidenced a statistically significant difference in survival between residual tumor with stromal invasion > and <3 mm. Therefore, a cut-off of 3 mm of residual stromal invasion seems to outperform all other residual tumor scoring systems for prognostic stratification of post-neoadjuvant treatment cervical cancer [5]. Moreover, the objectivity of the measurement of the depth of stromal invasion makes this system heavily reproducible with limited inter-observer variability.2.7. Maximum Horizontal Extent of Tumor
The horizontal extent of the tumor represents the longitudinal extent if the tumor is measured in the superior–inferior plane, or the circumferential extent if the tumor is measured perpendicular to the longitudinal axis of the cervix. It is best calculated histologically for smaller neoplasms or grossly for larger tumors [4]. Despite literature data suggesting its potential role as independent predictor of survival in cervical carcinoma, it is no longer utilized to stage microscopic (Stage IA) disease [4][56][4,56]. Therefore, the horizontal extent of the tumor is now considered as an optional element and its inclusion in the pathology report is encouraged to:- -
-
give a more complete picture of tumor extent (length and width);
- -
-
appreciate tumor volume;
- -
-
help future studies to further clarify its prognostic role.
