Biosurfactants are amphipathic molecules capable of lowering interfacial and superficial tensions. Produced by living organisms, these compounds act the same as chemical surfactants but with a series of improvements, the most notable being biodegradability. Biosurfactants have a wide diversity of categories. Within these, lipopeptides are some of the more abundant and widely known.
- biosurfactant
- lipopeptide
- protein-containing biosurfactant
- biosurfactant physicochemical properties
1. Introduction
2. Biosurfactant-Producing Microorganisms
2.1. Lipopeptides
Lipopeptides are a class of biosurfactants with high industrial interest. They are linear or cyclic oligopeptides acylated with fatty acids of different length and composition which are synthesized by the nonribosomal peptide synthetases (NRPSs) [16][62] and commonly secreted by bacterial genres such as Bacillus, Streptomyces and Pseudomonas [14][25]. Microorganisms of the genus Bacillus are some of the more well-known lipopeptide producers, and their genome contains a large operon (srfA) composed of four open reading frames which encode a peptide synthetase responsible for the surfactin biosynthesis (Figure 1A,B) [17][18][63,64]. Lipopeptides have varying emulsification properties according to temperature, pressure, pH, structure and stability of the solution [19][65]. For example, the emulsifying activity of surfactin produced by B. subtilis can change based on pH. With a pH above 7, it forms a stable emulsion with kerosene, but when the pH drops to 3 the emulsion does not form [20][66].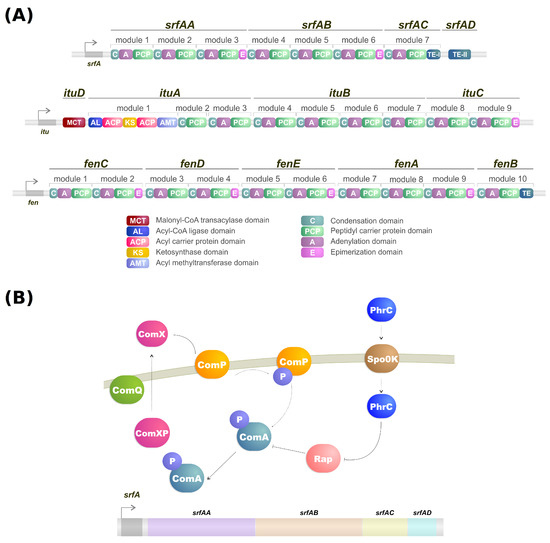
2.2. Protein-Containing Biosurfactants
3. Lipopeptides Synthesis and Regulation
NRPSs are multidomain mega-enzymes that synthesize NRPs without using the ribosomal machinery [24][84]. They have a modular structure in which each part incorporates an amino acid to the peptide moiety of the lipopeptide (Figure 1B). Another characteristic is that the NRPS obeys the colinearity rule; that is, the modules are colinear with the amino acid sequence of the peptide [16][62]. There are two types of modules: initiation and elongation modules. Each of these modules also contains domains that perform specific tasks [16][62]. While typically, initiation modules have domains responsible for the amino acid election, activation, and thioesterification of the activated amino acid, the first module also contains a condensation domain in lipopeptide biosynthesis. This domain is responsible for catalyzing the N-acylation of the first amino acid of the lipopeptide, thus linking the lipid moiety to the oligopeptide [16][25][62,85]. Elongation modules contain the same domains, but this time the condensation domain is responsible for catalyzing the peptide bond between two amino acids. The condensation domain from the elongation module will generate a lipopeptide that, by the end of the assembly line, will be cleaved by a thioesterase [16][62].
It has been shown via analyses of the metabolic profiles of Pseudomonas and Bacillus species that a single strain may be able to produce different forms of the same biosurfactant [16][62]. Examples include B. subtilis strain OKB 105, which can produce 12 different surfactin analogs [26][86] and P. fluorescens strain SS101 that can produce up to eight analogs of the lipopeptide massetolide A [27][87]. These analogs are thought to be the product of flexibility in the amino acid selection and activation by the adenylation domain of the initiation module in NRPSs. Said flexibility is common in nonribosomal peptide synthesis, which may have biological purposes for the producers [16][62].
Iturin production has a series of regulation factors. For example, the methylation of tyrosine residues in iturin can decerease yield and antibacterial activity [28][29][91,92]. Furthermore, iturin can also be regulated by controlling the expression of sigma factor A and the transcription factor ComA. Overexpressing the genes sigA and comA increased iturin yield [30][93].
As mentioned, the biosynthesis of surfactin is coordinated by the srfA operon, which contains four open reading frames (srfAA, srfAB, srfAC, and srfAD) as seen in Figure 1A [31][94]. These open reading frames are responsible for the peptide chain extension, a key step in the surfactin synthesis regulation [29][92]. Surfactin, much like iturin, is also strongly regulated by the transcription factor ComA, which binds to srfA, and thus controls its transcription and regulates srfA expression (Figure 1B) [29][92]. ComA phosphorylation is the key to activating the srfA operon transcription by two different pathways. The first involves the ComX peptide modified by ComQ that stimulates ComP autophosphorylation, triggering ComA phosphorylation (Figure 1A) [32][43]. Activated ComA translocates to the nucleus and promotes srfA transcription. PhrC importation is mediated by Spo0K, which interacts with Rap protein and inhibits its phosphatase activity, thus, preventing ComA dephosphorylation and facilitating ComA-induced srfA transcription (Figure 1A) [32][33][43,95].
4. Biosurfactant Toxicity
Most chemically synthesized surfactants widely available on the market resist biodegradation and accumulate in nature. On the other hand, Biosurfactants are, like all-natural products, susceptible to degradation in water and soil [6][14]. Other than that, they are known for being less toxic or even non-toxic to the environment when compared to their synthetic counterparts and, thus, being the better choice when degrading pollutants. Some studies were already published comparing synthetic and natural surfactants and show how biosurfactants can be less toxic or even non-toxic at all [34][35][105,106]. However, Edwards et al. (2003) determined that the difference may not be significant [36][107]. Still, there are issues regarding the conditions in nature and the concentration and availability of biosurfactants in said situations, as they can vary between species and compounds, and lab conditions cannot properly mimic that.5. Emerging Strategies for Biosurfactant Production
Being able to implement the newly discovered compounds is needed. As mentioned before, biosurfactants still struggle to compete with synthetic surfactants on large-scale applications globally. With that in mind, recent strategies have been developed to turn biosurfactants into competitive alternatives for industrial applications. One such application is solid-state fermentation (SSF). A strategy for biosurfactant production that is geared towards overcoming the foaming encountered in the more popular submerged fermentation (SmF), this is used not only for biosurfactants but also other bioproducts [37][38][39][112,113,114]. This methodology has already been applied to the peptidic biosurfactant surfactin,. By using a medium based on okara, with the addition of sugarcane bagasse as a bulking agent, SSF was employed for surfactin production by B. pumilus UFPEDA 448 [40][115]. Other examples include SSF of soybean flour and rice straw as a substrate for the production of an antimicrobial lipopeptide by B. amyloliquefaciens XZ-173 [41][116], lipopeptide production by B. subtilis SPB1 grown on a mixture of olive leaf residue flour and olive cake flour [42][117] and surfactin production via SSF with rapeseed cake mixed with bacterial solution [43][118]. The market always favors lucrative processes, and with that in mind, another feasible option for biosurfactant production is the co-production with other economically necessary products in a single bioprocess. Microorganisms tend to synthesize biosurfactants and other compounds, which could be taken advantage of. Before, co-production of pectinase and biosurfactant was observed in a B. subtilis strain isolated from a fruit dump yard [44][123].6. Physical and Chemical Properties of Biosurfactants
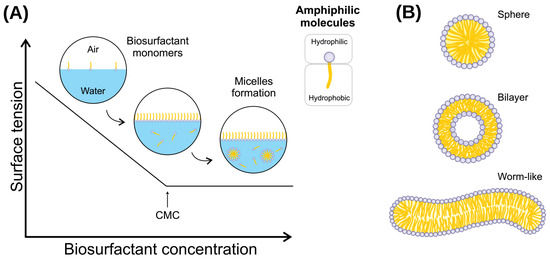
Biosurfactants are bioderived or biomimetic surfactants that can act as detergents, wetting agents, emulsifiers, dispersants, and foaming agents [45][46][126,127]. These molecules have the properties of reducing water surface tension and decreasing water/oil interface tension (Figure 2) [3]. This occurs because these amphiphilic molecules can dispose of the limit of surface water and assemble into micelles, which can shield hydrophobic molecules present in the solution from the unfavorable interactions with water molecules [19][65]. Moreover, biosurfactants present adsorptive properties on surfaces and interfaces [47][128].