Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Raffaele Serra and Version 3 by Dean Liu.
Vascular graft or endograft infection (VGEI) is a complex disease that complicates vascular-surgery and endovascular-surgery procedures and determines high morbidity and mortality.
- VGEI
- infection
- vascular prosthesis
- vascular graft
1. Introduction
A vascular prosthesis is defined as a biological or artificial device meant to replace a segment of an arterial tree whose function is compromised by injury, occlusive disease, or aneurysmal degeneration. A prosthetic graft provides a substitute conduit for blood flow, allowing the diseased vessel segment to be repaired, excised, or bypassed [1][2][3][4][5][6][7][8][9][10][1,2,3,4,5,6,7,8,9,10].
Vascular prostheses include vascular grafts (VGs), generally implanted surgically, and vascular endografts (VEs) (or stent-grafts) implanted by endovascular procedure. VGs may be classified into biological grafts, which are composed of actual tissues, most often blood vessels (e.g., autologous grafts derived from the patient’s own vessel); allografts (from human vessels); xenografts (generally of bovine origins); and synthetic grafts made from either poly-ethylene-terephthalate (PET, or Dacron), a textile material, or expandedpolytetrafluoroethylene (ePTFE), a non-textile material. In textile VGs, the basic polymer is first made into a yarn, which is then used to construct a graft using various methods of knitting or weaving. Non-textile VGs are manufactured using the techniques of the precipitation or the extrusion of the polymer from solutions or sheets of the material. When the available length of an autogenous tissue graft is inadequate for the required reconstruction, composite VGs can be used. Such VGs are constructed by combining segments of an artificial prosthetic material with autogenous material to form a substitute-vessel conduit. VGs are generally positioned during open surgery and sutured both proximally and distally to the healthy artery by end-to-end or end-to-side anastomosis (Figure 1). An ideal VG should be impermeable, thromboresistant, compliant, biocompatible, durable, resistant to infection, easy to sterilize, easy to implant, readily available, and cost-effective. In particular, biocompatibility is necessary because significant tissue reaction may promote thrombosis, loss of graft integrity, and graft failure. Sterility and resistance to infection are necessary to decrease the incidence of infection. VEs are transluminally implanted vascular devices introduced into the vascular system via a remote artery using minimally invasive techniques that combine a prosthetic fabric with a vascular stent (Figure 1). Vascular stents are made by different alloys, with nitinol being the most used. The graft is anchored in place by a balloon-expandable or self-expanding metal frame that supports all or part of the graft and provides a tight seal proximal and distal to the diseased segment of the artery. Since it circumvents the need for laparotomy, the cross-clamping of the artery, and the obligatory blood loss associated with the opening of the aneurysm sac, the technique has been shown to reduce the morbidity and mortality associated with conventional surgery, and it expands the patient pool to include patients with severe medical- and co-morbidities who were previously denied treatment [11][12][13][14][15][16][17][18][19][20][21][22][11,12,13,14,15,16,17,18,19,20,21,22].
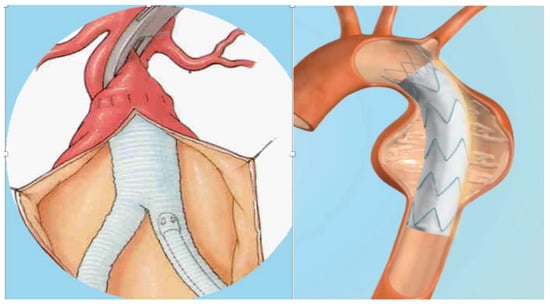
Figure 1. Vascular graft and vascular endograft. On the (left): vascular graft; on the (right): vascular endograft.
According to the location of implantation, grafts may be extra-cavitary (groins and lower limbs) and intra-cavitary (abdominal or thoracic aorta) [21][22][21,22].
Vascular prostheses may be burdened by a series of complications mainly due to fabrication or biomaterial failure such as dilation, rupture, thrombosis, allergic foreign-body reaction, and infection. In particular, a vascular graft or endograft infection (VGEI) is a clinically important complication that may occur following VG surgery or VE procedures accompanied by high morbidity and mortality rates [22][23][24][25][26][27][28][29][30][31][32][33][22,23,24,25,26,27,28,29,30,31,32,33]. The incidence of thoracic aortic VGEI is around 6% and, with mortality rates that relate to the clinical presentation, around 75%. The incidence of VGEI in the abdominal aorta is a rare complication, occurring in <1%, but one with a high mortality rate. VGEIs in peripheral-artery surgery are mainly represented by vascular graft infection (VGI) for open surgery with an incidence of up to 2.8% [22]. The percentage of infection in prosthetic arteriovenous hemodialysis grafts (AVHGs) is approximately 3.5% [34]. VGEIs are managed mainly through surgical removal, revascularization, and long-term antibiotic treatment. The explantation of an infected graft may determine important mortality rates (18–30%), while conservative management with long-term antibiotic therapy may result in a very high mortality rate, reaching about 100%, if the VGEI is not completely resolved [21].
VGEI, although not particularly frequent, is a multifaceted disease, and diagnosis may be challenging and even complicated for physicians, as they sometimes may be not as timely as an optimal treatment requires. Considering the rarity of this disease, current literature is not specialized as it lacks randomized controlled trials and studies with high patient population. Furthermore, current clinical evidence is based on small case studies, which are very often retrospective in nature [35].
2. Prevention of VGEI
The prevention of VGEI generally consist of adequate pre-operative patient preparation, heedful surgical and procedural management, antisepsis measures, prompting the administration of pre-operative antibiotic prophylaxis, and adequate wound care [34]. Before surgical procedures, it is important to treat any potential source of infection (i.e., dental problems, etc.) [22][36][22,58]. In the context of preoperative patient preparation, it seems reasonable to screen patients undergoing graft implantation for S. aureus nasal carriage and, if positive, provide peri-operative nasal eradication therapy because of the risk of potentially related severe VGEIs [22][37][22,105]. Adequate antimicrobial prophylaxis with broad-spectrum systemic antibiotics seems to significantly reduce the risk of early graft infection. Generally, the first or second generation of cephalosporin is used. It is also important that antimicrobial prophylaxis covers the most frequent bacteria, including MRSA, and for institutions with high rates of MRSA, daptomycin or vancomycin can be administered additionally [22][34][22,34]. Hair removal and appropriate aseptic care in the operating theater remain pivotal in peri-operative care [22]. Postoperative measures consist of speeding up the healing of surgical wounds, for example, the use of negative-pressure wound therapy (NPWT), whereby applying sub-atmospheric pressure decreases inflammatory exudates promoting granulation tissue, may be used to achieve fast wound closure, [38][39][40][41][42][106,107,108,109,110] and to consider antimicrobial prophylaxis before any dental procedure [22][43][44][45][46][47][22,111,112,113,114,115].3. Treatment of VGEI
The best treatment for VGEI mainly depends on the location of the graft, the extent of the infection, and the type of microorganism. Generally, the management plan includes the removal of the graft, a careful debridement of the infected surrounding tissues, the restoration of circulation distal to the GI, and adequate antibiotic therapy [36][58]. Figure 2 and Figure 3 show an infected VG and VE, respectively, during explantation procedures.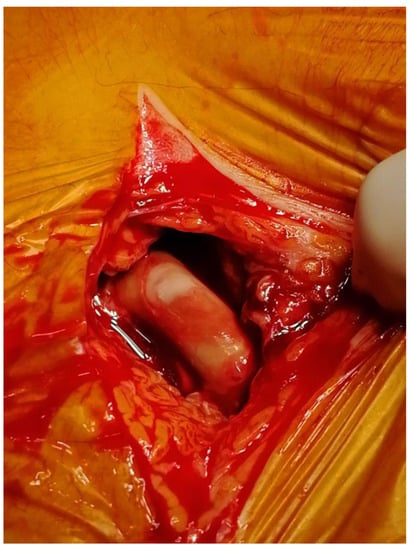
Figure 2. Explantation of an infected vascular graft at the groin.
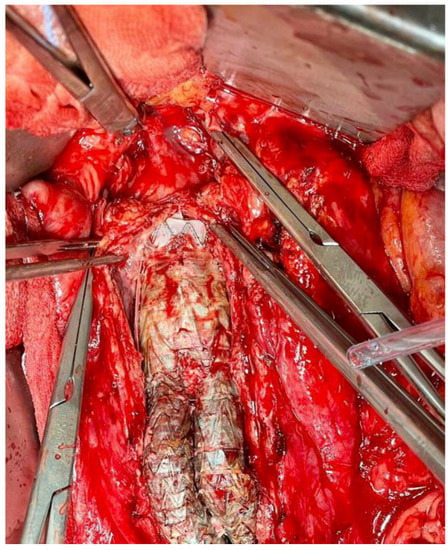
Figure 3. Explantation of an infected aortic and iliac endograft.
Figure 4 shows a general evidence-based algorithm in a case of VGEI.
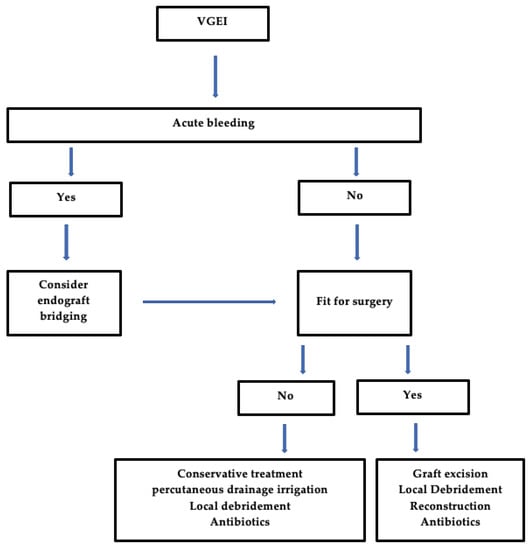
Figure 4. General Algorithm for the management of VGEI. VGEI = vascular graft or endograft infection.
