Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Olivia Costantina Demurtas.
Contrary to the biosynthetic pathways of many terpenoids, which are well characterized and elucidated, their transport inside subcellular compartments and the secretion of reaction intermediates and final products at the short- (cell-to-cell), medium- (tissue-to-tissue), and long-distance (organ-to-organ) levels are still poorly understood, with some limited exceptions.
- terpenoids
- carotenoids
- apocarotenoids
- ABA
- strigolactones
1. Introduction
Plant specialized metabolites (PSMs), previously referred as secondary metabolites, are small molecules (<2000 Da) synthesized and accumulated in specific tissues and developmental stages and/or under particular environmental conditions, which own different functions aiming at generally improving plant fitness, including physiological aspects and responses to biotic and abiotic stresses [1]. These metabolites are generally produced in specific organs, cells, or subcellular compartments that are tightly subjected to intra- or inter-cellular transport through biological membranes. Several transport mechanisms have been described and characterized in past years for many classes of PSMs, such as alkaloids, phenylpropanoids, and terpenoids. Among these classes of compounds, terpenoids, also referred as isoprenoids, are the most diverse, with about 80,000 distinct compounds that have important implications in plant growth, development, and adaptation, and in interaction with other organisms [2,3,4][2][3][4]. Relatively few isoprenoids have essential roles in plant development and fitness, such as carotenoids, sterols, gibberellins, chlorophylls, and plastoquinones [2,3,5][2][3][5]. The vast majority of plant terpenoids are PSMs involved in plant–environment interactions [6,7][6][7]. For example, diverse terpenoids, including carotenoids, their derivatives, and volatile terpenoids, have indispensable functions in attracting pollinators and seed dispersers [3[3][8][9],8,9], while others have roles in mediating beneficial symbiotic relationships that improve plant fitness [10]. Terpenoids also take part in allelopathic interactions and in abiotic stress responses [11,12][11][12]. Many terpenoids are instead potent toxic compounds that serve as chemical defenses against herbivores, insects, and microbial pathogens [2,3,13][2][3][13]. Together with the expansion in terpenoid metabolism, which takes place when land plants face environmental changes and biotic or abiotic stresses [3[3][5],5], several mechanisms to improve their differential compartmentation, secretion, and bioactivity have been evolved.
2. General Transport Mechanisms for PSMs
The transport of molecules between and within cells generally follows different types of mechanisms, based on the nature of the compound. In more detail, gasses and some small lipophilic compounds, including a few volatiles, can pass through membranes by simple diffusion [17][14], while charged or bigger molecules, such as metals, ions, sugars, vitamins, volatiles, and general/specialized metabolites, need a protein- or lipid-mediated translocation or vesicle-mediated transport [14,18,19,20,21][15][16][17][18][19]. Within cells, the intracellular trafficking of vesicles that bud off from the endoplasmic reticulum (ER) allows the secretion of proteins, lipids, components of the membranes, and other molecules into the plasma membrane and apoplast, or to subcellular compartments, such as vacuole and peroxisomes [18,22,23,24][16][20][21][22]. The transport is basically obtained by the fusion of vesicles to cellular membranes, with the release of their content. Besides vesicle-mediated transport, the main mechanism responsible for PSMs is protein-mediated transport across membranes [25,26][23][24]. The transmembrane transporters generally involved in this process belong to five families: (i) ATP-binding cassette (ABC) transporters, (ii) multidrug and toxin extrusion (MATE) proteins, (iii) nitrate-peptide transporter (NPF), (iv) purine uptake permease (PUP), and (v) AWPM-19 family proteins [14,25,27][15][23][25]. Among them, only ABC transporters mediate the primary active transport (e.g., the transport is driven by the hydrolysis energy of ATP), while the others mediate the secondary active transport (transport driven by an electrochemical gradient). Overall, the different transporter classes are described as follows:-
ABC transporters function as ATP-driven pumps to import and export molecules of diverse types, such as metals, xenobiotics, hormones, pigments, and largely diversified specialized metabolites [16,28][26][27]. The ABC family is highly expanded in the plant kingdom in comparison to other organisms, and in Arabidopsis thaliana many members have been functionally characterized [15][28]. This expansion is mainly due to the fact that plants are sessile organisms that need to adapt to environmental changes and modulate the expression of genes involved in the synthesis, storage, and release of specific metabolites that serve to cope with the modified conditions [29]. In general, nine subfamilies of ABC transporters have been identified in plants (from ABCA to ABCI, with the exception of the ABCH proteins that have not been found yet) [30], with the B, C, and G subfamilies being the most abundant. The members of these subfamilies have been described as being involved in the translocation of PSMs, toxic compounds, and phytohormones [15][28], molecules that are particularly important for the selective advantage of plants to terrestrial life during evolution [31]. ABCGs and ABCBs are plasma membrane-localized ABC transporters responsible for the translocation of PSMs between cells, while ABCCs are generally present in the tonoplast, where they contribute to the vacuolar accumulation of metabolites. More particularly, the ABCG subfamily, also called pleiotropic drug resistance (PDR) transporters, is one of the predominant class of transporters involved in important biological functions, such as pathogen defense, abiotic stress tolerance, cuticular formation, and phytohormone transport [14,15,32][15][28][32]. For a detailed and updated status of ABC transporter proteins in plants, see [16][26].
-
MATEs are secondary transporters that use proton or sodium ion gradients to translocate substrates across membranes [33,34][33][34]. Like ABC transporters, MATEs have been subjected to a high expansion through tandem and segmental duplication events during the course of evolution. In Arabidopsis, 58 members of MATE transporters, also called DETOXIFICATION (DTX) proteins, have been identified [34]. MATEs exhibit a wide array of functions in plants, including detoxification of xenobiotics and transport of metals, phytohormones, and PSMs [14,25][15][23]. Four main classes of MATEs have been identified to date [33]; many MATE members are located in the plasma membrane and function as exporters, but several tonoplast members have also been identified and described as transporters for the vacuolar sequestration of PSMs (especially alkaloids and phenylpropanoids) [21,35,36,37][19][35][36][37].
-
NPFs, previously known as NRTs, use proton gradient to transport different classes of compounds such as nitrate, amino acids, peptides, phytohormones, and PSMs [25][23]. After the first discovery of a NPF as a nitrate transporter in A. thaliana [38], NPFs were found to translocate amino acids and peptides [39,40][39][40]. Lately, it was demonstrated that NPFs are responsible for the transport of glucosinolates and alkaloid derivatives [41,42,43,44,45,46][41][42][43][44][45][46]. To date, eight subfamilies of NPFs have been identified [43] based on the nomenclature proposed in [47].
-
For several years, PUP transporters were thought to be involved in the translocation of purine nucleobase substrates. However, in 2011, it was demonstrated that a Nicotiana tabacum PUP-like homolog, named NUP1, is involved in the transport of alkaloid nicotine across the plasma membrane [48]. These findings pave the way for the study of PUP-like proteins as PSM transporters. Two other studies displayed that PUP-like proteins are involved in the transport of a few other alkaloids [49,50][49][50]. Interestingly, the PUP-like family originated during terrestrial plant evolution between the bryophytes and the lycophytes. A phylogenetic study showed an extensive pattern of gene duplication and diversification within the angiosperm lineage, with sub-functionalization and neo-functionalization of PUP-like transporters [51].
-
More recently, a new class of transporters, belonging to the AWPM-19 family, has been identified in wheat and rice, albeit they have been also described in Arabidopsis [27,[52,2553]][52][53]. AWPM-19 transporter proteins are encoded by an ancient, highly conserved member of the plasma membrane 4 gene family, and they have been found to play a role in abscisic acid (ABA) transport. Actually, in species, such as rice, in which no authentic ABC-type ABA transporters have been identified, the OsPM1 AWPM-19 member has been considered the main transporter responsible for ABA import and drought-related responses [27][25].
3. Function and Transport of Terpenes
3.1. Generalities
Terpenes constitute a large and structurally diverse family of primary and specialized metabolites in plants [2,5][2][5]. Some terpenes are produced in low amounts and serve as plant phytohormones, such as gibberellins (GAs), cytokinins (CKs), abscisic acid (ABA), and strigolactones (SLs); others, such as carotenoids, are produced in bulk amounts and have a role as light-harvesting pigments and photoprotectors in photosynthesis, or an ecological role as pigments to attract pollinators and seed dispersers. In addition, plants contain a huge variety of monoterpenes, sesquiterpenes, diterpenes, triterpenes, and carotenoid derivatives (apocarotenoids) that function as specialized metabolites with important ecological functions in the interaction of plants with other organisms [6,7,67,68,69][6][7][67][68][69]. Terpenes have simple hydrocarbon structures, while terpenoids present different functional groups [2]. Numerous terpenoids are bioactive molecules that are used in medicine (i.e., taxol or artemisinin) or as flavor and fragrance compounds [70,71,72][70][71][72]; therefore, there is a great interest on the elucidation and engineering of the biosynthetic pathways involved in their transport within or between cells.3.2. Biosynthesis and Functions
Terpenes can be classified, based on the number of the five-carbon isoprene units, as hemiterpenes (C5), monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), sesterpenes (C25), triterpenes (C30), and tetraterpenes (C40). They are all synthesized from the condensation of the five-carbon isoprenoid precursors, isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). IPP and DMAPP are generated by two independent, cell-compartmentalized biosynthesis: the cytosolic mevalonic acid (MVA) pathway, and the plastidial methylerythritol phosphate (MEP) pathway [5]. In the MVA pathway, the precursors derived from acetyl-CoA are condensed by farnesyl diphosphate synthase (FPS) to form farnesyl diphosphate (FPP, C15), which is the precursor of sesquiterpenes, triterpenes, and sterols. On the contrary, in the MEP pathway, IPP and DMAPP are originated from pyruvate and glyceraldehyde-3-phosphate and are condensed by geranyl diphosphate synthase (GPS) to form geranyl diphosphate (GPP, C10), which is the direct precursor of monoterpenes, and by geranylgeranyl diphosphate synthase (GGPPS) to form geranylgeranyl diphosphate (GGPP, C20), which acts as a precursor for diterpenes and carotenoids [5,73][5][73]. After the formation of the precursors, FPP, GPP, and GGPP, the different terpenes are generated through the action of terpene synthases (TPS), which are enzymes implicated in the formation of primary terpene skeletons, and are then further modified by the action of various enzyme classes, such as cytochrome P450 hydroxylases, dehydrogenases, and glycosyl- and methyl-transferases [6,73][6][73]. These enzymes have various subcellular localization: for instance, P450 enzymes are generally located in the ER membrane [74], while other enzymes are placed essentially in the cytosol or in the ER surface [75,76,77,78][75][76][77][78]. Differently from terpenes that are synthesized in the cytosol, such as tri- and sesquiterpenes, the biosynthesis of diterpenes and tetraterpenes (and their derivatives) implies the translocation of intermediates from the plastid to the cytosol and/or ER, where the modification enzymes reside. This kind of transport is poorly understood, and several pieces are missing in a puzzle which is still being solved (Figure 1). The final products of terpenoid biosynthesis are then (i) accumulated in the cytosol, (ii) stored in the vacuole, or (iii) secreted into the apoplast. Generally, monoterpenes and sesquiterpenes are secreted through the plasma membrane and accumulate in the apoplast or in specialized structures, such as oil gland or glandular trichomes [79,80][79][80]. These molecules have strong ecological roles as phytochemicals against pathogens and herbivore attacks [81,82][81][82] or as volatiles emitted for the attraction of pollinators and natural enemies of herbivores [83,84][83][84]. Additionally, they are the main constituents of essential oils and are of great interest for their biological properties, such as antioxidant, anticancer, anti-inflammatory, antimicrobial, antiviral, anthelminthic, antinociceptive properties [70,71,72,85,86,87][70][71][72][85][86][87]. Terpenoids also contribute to the formation of complex molecules, such as monoterpene indole alkaloids (MIAs), which are PSMs with powerful pharmacological activities (e.g., the well-known anti-tumor agents vinblastine and vincristine) [88,89][88][89]. Among sesquiterpenes, a well-known member is β-caryophyllene, a volatile compound present in many essential oils, especially from clove, rosemary, and Cannabis sativa, that plays an important role in the defense against microbial pathogens [90,91][90][91]. Triterpenes are the largest subgroup of structurally diverse terpene molecules that includes sterols, steroids, and saponins. Triterpenoids derived from squalene [92] and its members have fundamental roles as wax and resin components, e.g., lupeol and β-amyrin, which are also the precursors of pentacyclic metabolites with distinct bioactivity against plant biotic stressors, such as betulinic acid (from the bark of birch tree, Betula pubescens) or glycyrrhizin (found in the roots and stolons of licorice, Glycyrrhiza glabra), which are also characterized by their pharmaceutical and, limited to the latter, sweetener properties [93,94][93][94]. Diterpenes also have important functions as biotic and abiotic interactions, and some of them act as phytohormones (for example, gibberellins or GAs [78]). Finally, carotenoids are a large group of tetraterpene pigments, with more than 600 different structures [7,9,95][7][9][95]. They are produced in plastids and are the precursors of apocarotenoids, which are enzymatic or non-enzymatic cleavage products that are subjected to several modifications, stored in the vacuoles or secreted outside the cells, and play a role as signal molecules, pigments, aromas, or hormones [96,97,98,99,100][96][97][98][99][100].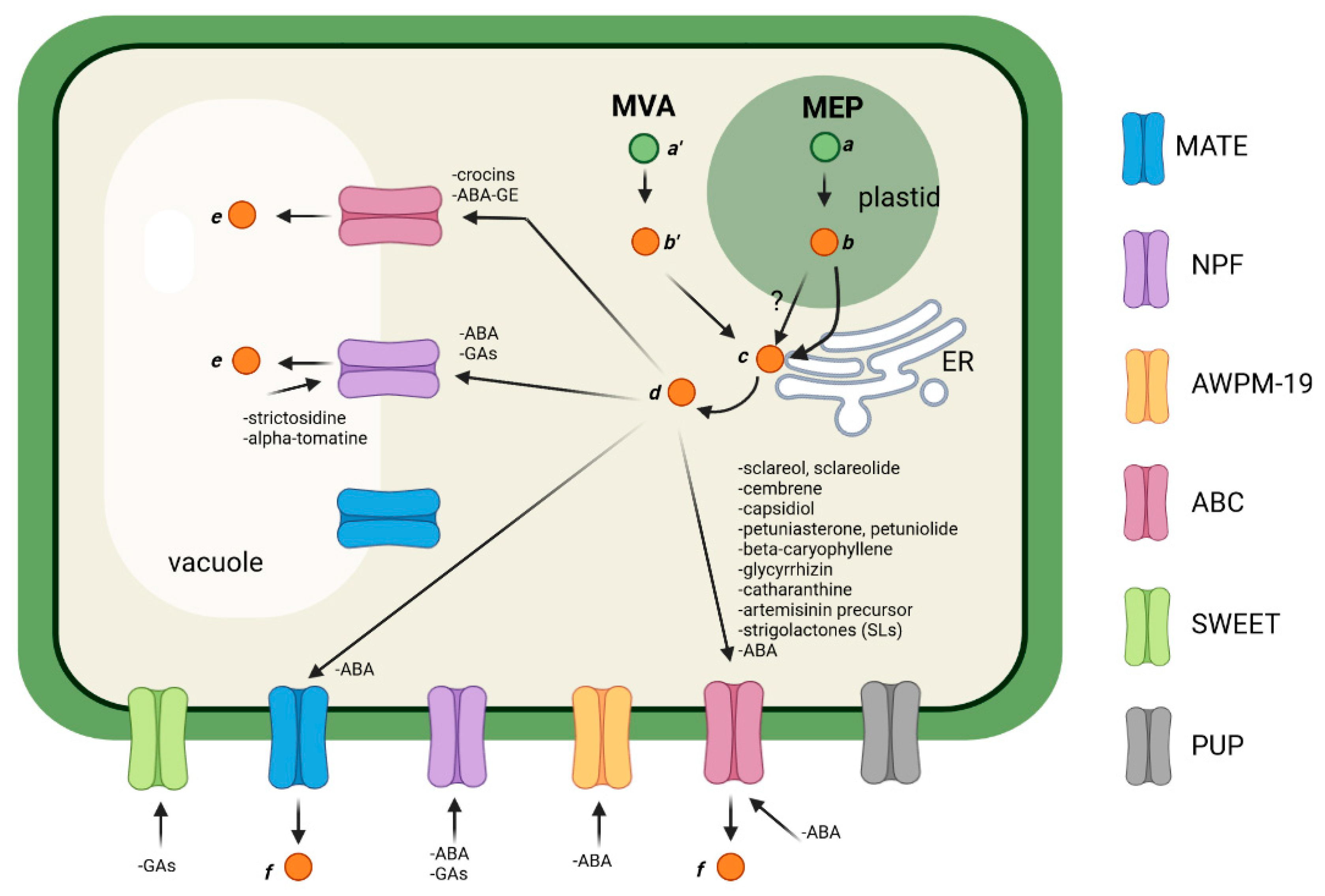
Figure 1. General scheme depicting the transport of terpenoids in plant cells.
3.3. Transport of C10-C15-C20-C30 Terpenoids (Mono-, Sesqui-, Di-, and Triterpenoids)
Although small volatile terpenes emitted by leaves, such as isoprene, are released by simple diffusion across biological membranes [108[108][109][110],109,110], bigger terpenes, including monoterpenes, generally require the presence of active transporters or vesicle-mediated transport [20,111][18][111]. For years, it was believed that volatile organic compounds (VOCs) cross membranes by diffusion; however, it was then demonstrated that lipophilic small compounds tend to accumulate into biological bilayers with detrimental effects on membrane integrity, demonstrating that different translocation mechanisms are involved [20,111][18][111]; for instance, in Petunia hybrida, an ABC transporter is responsible for the translocation of different VOCs across the plasma membrane [111]. Regarding terpenoids, in Vitis vinifera, different sesquiterpenoid volatiles are emitted in the flowers, and it has been demonstrated that the sesquiterpene synthase, valencene synthase (VvValCS), which is responsible for their synthesis, is localized to the outer edges of lipid vesicles in pollen grains [112]. Thus, this finding suggests that valencene is stored and secreted through lipid vesicles. Another study provided clues about the involvement of a vesicle-mediated transport of monoterpenes in the secretory cells of the glandular trichomes of Prostanthera ovalifolia. Indeed, the electron microscopy images show that plastids (where the biosynthesis of the precursor of monoterpenes takes place) are surrounded by vesicles that then fuse with the plasma membrane [113]. An additional work evidenced that a vesicle-mediated transport from the ER to the plasma membrane is involved in the subcellular movement of the sesquiterpenes copaene and β-caryophyllene in Sauromatum guttatum flowers [114]. β-Caryophyllene is a potential toxic volatile since, in the cytosol, it can react with proteins, leading to the formation of caryophyllene oxide (CPO) [115]. CPO induces increased reactive oxygen species (ROS) generation from mitochondria, leading to the induction of degenerative processes [112]. For this reason, the cells need to emit it in the headspace of the plant, and, here, it serves as a defense against bacterial pathogens that invade tissues [90,91][90][91]. Interestingly, for this compound, in addition to the vesicle-mediated transport, the involvement of an active transporter belonging to the ABCG subfamily has been demonstrated [81]. Plasma membrane-localized ABCGs are, in fact, the main class of transporters implicated in the secretion of terpenes [15,116][28][116]. For example, it has been shown that the exporter AaABCG3/AaPDR3 is responsible for β-caryophyllene secretion in Artemisia annua T-shaped trichomes and roots [81]. Of note, this transporter has high homology sequence with Nicotiana plumbaginifolia NpPDR1, N. tabacum NtPDR1, Arabidopsis thaliana AtPDR12, and Spirodela polyrrhiza SpTUR2 transporters [81]. The NpPDR1 of N. plumbaginifolia transports the antifungal diterpenes, sclareol and sclareolide, across the plasma membrane, resulting in their secretion on the glandular trichomes, where they function as antifungal compounds [117,118][117][118]. In contrast, the homolog of this transporter in N. tabacum, NtPDR1, is able to translocate not only sclareol, but also the diterpene cembrene [119] and the sesquiterpene capsidiol [120], a phytoalexin involved in the defense against pathogens. Additionally, the NbABCG1 and NbABCG2 transporters of N. benthamiana seem to be involved in the same transport [121[121][122],122], and an ortholog of the NpABC1 transporter has also been found in Arabidopsis, AtPDR12 [123] and S. polyrhiza, SpTUR2 [124]. Notably, SpTUR2 was the first plant PDR transporter to be characterized [125], and its expression in Arabidopsis plants leads to the acquisition of resistance to sclareol [124]. In P. hybrida, for instance, it was shown that the PhPDR2 transporter is involved in the defense against herbivores, secreting two steroidal-derived compounds (petuniasterone and petuniolide) [126]. Another well-known example is artemisinin, a potent anti-malarial sesquiterpene lactone that accumulates in the glandular trichomes of Artemisia annua [127]. Indeed, Wang and coauthors [128] demonstrated that two transporters from A. annua, the AaPDR2 transporter and the lipid-transfer protein 3 (AaLTP3), once expressed in N. benthamiana leaves, led to the accumulation of the precursor of artemisinin, (DH)AA (dihydro)artemisinic acid [DHAA]), that is then photochemically converted to artemisinin in the apoplastic space, suggesting the involvement of these transporters in vivo. Finally, a recent study reported the identification of four members of plasma membrane-localized ABCG transporters in Salvia miltiorrhiza Bunge, a plant used in traditional Chinese medicine to treat cardiovascular and cerebrovascular diseases; these transporters could be potentially involved in the export of tanshinone (a lipophilic diterpene) and salvianolic acid (a hydrophilic phenolic compound), which are metabolites highly accumulated in the roots and rhizomes of S. miltiorrhiza [129]. Additionally, in the present Special Issue, Kato and coauthors described the transport of the triterpene saponin glycyrrhizin in Glycyrrhiza glabra (licorice plant) by a H+-symporter in the plasma membrane and by an ATP-binding cassette transporter in the vacuole (with a high specificity for the aglycone form) [130]. An additional example of the vacuolar transport of terpenoids is represented by avenacin A-1 and triterpene saponins of oat [131]. Avenacin A-1 is a glucosylated antifungal compound that is stored in the vacuole, and its transport has not yet been elucidated, albeit the co-authors suggested that an ABC transporter might be the main responsible. Some information is also available for the transport of MIAs. The majority of MIAs are derived from the assembly of tryptamine and the monoterpene secologanin to form the central intermediate strictosidine. The transporter responsible for the vacuolar export of this intermediate was identified in Catharanthus roseus: a NPF transporter, named CrNPF2.9, localizes to the tonoplast of leaf epidermal cells and translocates strictosidine from the vacuole to the cytosol [44]. Similarly, a Solanum lycopersicum NPF transporter called GORKY is responsible for the export of the steroidal glycoalkaloid α-tomatine and its derivatives from the vacuole to the cytosol [46]. Another characterized transporter involved in MIA translocation belongs to the ABC Family, G group. The plasma membrane CrTPT2 transporter from C. roseus is involved in the export of catharanthine to the leaf surface [132]. Regarding diterpene transport, different studies have shed light on the transport mechanisms of the hormone gibberellin (GA). GA biosynthesis starts in plastids, with the formation of the intermediate Ent-kaurene, then proceeds in the ER, and finishes in the cytosol, where different GAs are formed [78]. The mechanisms responsible for the release of the precursors from the plastid and the ER have not yet been identified, while much information regarding the GA movement across the plasma membrane is available. Indeed, GA moves between cells through different mechanisms, with the ion trap being the main mechanism that allows GAs to enter cells [78]. Following entrance into the cytosol, GAs are converted into their charged forms (due to the weak alkaline pH of 7.2), which prevents their further simple diffusion; thus, the export of GAs requires the activity of specific transporters [133]. While GA efflux transporters have not yet been identified, several influx transporters have been described [134]: it was demonstrated in vitro in yeast cells that different NPF transporters are able to transport diverse GAs, as well as ABA and other hormones, such as jasmonic acid (JA) [135,136][135][136]. In more detail, these studies showed that, of the 45 NPF transporters identified, several are able to import different types of GAs [136,137][136][137]. Another type of transporter that has been revealed to be involved in the influx of GAs belongs to the class of SWEET transporters. In particular, the A. thaliana AtSWEET13 and AtSWEET14 transporters have been shown to be able to transport GAs in different forms (active and non-active) [138]. In addition, in rice, a SWEET transporter, OsSWEET3a, was found to be involved in the transport of GAs [139]. Interestingly, GA transport by SWEET proteins evolved independently during plant evolution: in fact, in Arabidopsis, it arose from sucrose SWEET transporters, while in rice, it arose from glucose SWEETs [139]. GAs are also accumulated in the vacuole, especially as a reservoir to use during the suberization process. It was demonstrated that GAs, as well as ABA, are loaded into the pericycle vacuoles at the phloem in the roots of A. thaliana to form a storage pool, which is later released into the endodermis to induce suberization [140]. An NPF transporter, named NPF2.14, is a tonoplast-localized transporter in pericycle cells able to transport GAs and ABA from the cytosol to the vacuole. When the root elongates and GAs and ABA levels in the vacuole become high, the hormones are exported out of the pericycle vacuole and imported into the endodermis by the NPF3.1 transporter [140].References
- Marone, D.; Mastrangelo, A.M.; Borrelli, G.M.; Mores, A.; Laidò, G.; Russo, M.A.; Ficco, D.B.M. Specialized metabolites: Physiological and biochemical role in stress resistance, strategies to improve their accumulation, and new applications in crop breeding and management. Plant Physiol. Biochem. 2022, 172, 48–55.
- Boncan, D.A.T.; Tsang, S.S.K.; Li, C.; Lee, I.H.T.; Lam, H.M.; Chan, T.F.; Hui, J.H.L. Terpenes and Terpenoids in Plants: Interactions with Environment and Insects. Int. J. Mol. Sci. 2020, 21, 7382.
- Karunanithi, P.S.; Zerbe, P. Terpene synthases as metabolic gatekeepers in the evolution of plant terpenoid chemical diversity. Front. Plant Sci. 2019, 10, 1166.
- Christianson, D.W. Structural and chemical biology of terpenoid cyclases. Chem. Rev. 2017, 117, 11570–11648.
- Zhou, F.; Pichersky, E. More is better: The diversity of terpene metabolism in plants. Curr. Opin. Plant Biol. 2020, 55, 1–10.
- Pichersky, E.; Raguso, R.A. Why do plants produce so many terpenoid compounds? New Phytol. 2018, 220, 692–702.
- Zheng, X.; Giuliano, G.; Al-Babili, S. Carotenoid biofortification in crop plants: Citius, altius, fortius. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158664.
- Jia, K.-P.; Baz, L.; Al-Babili, S. From carotenoids to strigolactones. J. Exp. Bot. 2018, 69, 2189–2204.
- Rosas-Saavedra, C.; Stange, C. Biosynthesis of carotenoids in plants: Enzymes and color. Carotenoids Nat. Biosynth. Regul. Funct. 2016, 79, 35–69.
- Fiorilli, V.; Wang, J.Y.; Bonfante, P.; Lanfranco, L.; Al-Babili, S. Apocarotenoids: Old and new mediators of the arbuscular mycorrhizal symbiosis. Front. Plant Sci. 2019, 10, 1186.
- Ninkuu, V.; Zhang, L.; Yan, J.; Fu, Z.; Yang, T.; Zeng, H. Biochemistry of terpenes and recent advances in plant protection. Int. J. Mol. Sci. 2021, 22, 5710.
- Rosenkranz, M.; Chen, Y.; Zhu, P.; Vlot, A.C. Volatile terpenes—Mediators of plant-to-plant communication. Plant J. Cell Mol. Biol. 2021, 108, 617–631.
- Verdeguer, M.; Sánchez-Moreiras, A.M.; Araniti, F. Phytotoxic effects and mechanism of action of essential oils and terpenoids. Plants 2020, 9, 1571.
- Wang, L.; Erb, M. Volatile uptake, transport, perception, and signaling shape a plant’s nose. Essays Biochem. 2022, 66, 695–702.
- Gani, U.; Vishwakarma, R.A.; Misra, P. Membrane transporters: The key drivers of transport of secondary metabolites in plants. Plant Cell Rep. 2021, 40, 1–18.
- Pucker, B.; Selmar, D. Biochemistry and molecular basis of intracellular flavonoid transport in plants. Plants 2022, 11, 963.
- Wang, S.C.; Davejan, P.; Hendargo, K.J.; Javadi-Razaz, I.; Chou, A.; Yee, D.C.; Ghazi, F.; Lam, K.J.K.; Conn, A.M.; Madrigal, A.; et al. Expansion of the Major Facilitator Superfamily (MFS) to include novel transporters as well as transmembrane-acting enzymes. Biochim. Biophys. Acta. Biomembr. 2020, 1862, 183277.
- Widhalm, J.R.; Jaini, R.; Morgan, J.A.; Dudareva, N. Rethinking how volatiles are released from plant cells. Trends Plant Sci. 2015, 20, 545–550.
- Biała, W.; Jasiński, M. The Phenylpropanoid Case—It is transport that matters. Front. Plant Sci. 2018, 9, 1610.
- Agaoua, A.; Bendahmane, A.; Moquet, F.; Dogimont, C. Membrane trafficking proteins: A new target to identify resistance to viruses in plants. Plants 2021, 10, 2139.
- Aniento, F.; Sánchez de Medina Hernández, V.; Dagdas, Y.; Rojas-Pierce, M.; Russinova, E. Molecular mechanisms of endomembrane trafficking in plants. Plant Cell 2021, 34, 146–173.
- Bassham, D.C.; Brandizzi, F.; Otegui, M.S.; Sanderfoot, A.A. The secretory system of Arabidopsis. Arab. Book 2008, 6, e0116.
- Nogia, P.; Pati, P.K. Plant secondary metabolite transporters: Diversity, functionality, and their modulation. Front. Plant Sci. 2021, 12, 758202.
- Shitan, N. Secondary metabolites in plants: Transport and self-tolerance mechanisms. Biosci. Biotechnol. Biochem. 2016, 80, 1283–1293.
- Yao, L.; Cheng, X.; Gu, Z.; Huang, W.; Li, S.; Wang, L.; Wang, Y.F.; Xu, P.; Ma, H.; Ge, X. The AWPM-19 Family Protein OsPM1 mediates abscisic acid influx and drought response in rice. Plant Cell 2018, 30, 1258–1276.
- Do, T.H.T.; Martinoia, E.; Lee, Y.; Hwang, J.-U. 2021 update on ATP-binding cassette (ABC) transporters: How they meet the needs of plants. Plant Physiol. 2021, 187, 1876–1892.
- Lefèvre, F.; Boutry, M. Towards identification of the substrates of ATP-binding cassette transporters. Plant Physiol. 2018, 178, 18–39.
- Banasiak, J.; Jasiński, M. ATP-binding cassette transporters in nonmodel plants. New Phytol. 2022, 233, 1597–1612.
- Hwang, J.U.; Song, W.Y.; Hong, D.; Ko, D.; Yamaoka, Y.; Jang, S.; Yim, S.; Lee, E.; Khare, D.; Kim, K.; et al. Plant ABC transporters enable many unique aspects of a terrestrial plant’s lifestyle. Mol. Plant 2016, 9, 338–355.
- Lane, T.S.; Rempe, C.S.; Davitt, J.; Staton, M.E.; Peng, Y.; Soltis, D.E.; Melkonian, M.; Deyholos, M.; Leebens-Mack, J.H.; Chase, M.; et al. Diversity of ABC transporter genes across the plant kingdom and their potential utility in biotechnology. BMC Biotechnol. 2016, 16, 47.
- Wang, S.; Li, L.; Li, H.; Sahu, S.K.; Wang, H.; Xu, Y.; Xian, W.; Song, B.; Liang, H.; Cheng, S.; et al. Genomes of early-diverging streptophyte algae shed light on plant terrestrialization. Nat. Plants 2020, 6, 95–106.
- Gräfe, K.; Schmitt, L. The ABC transporter G subfamily in Arabidopsis thaliana. J. Exp. Bot. 2020, 72, 92–106.
- Nimmy, M.S.; Kumar, V.; Suthanthiram, B.; Subbaraya, U.; Nagar, R.; Bharadwaj, C.; Jain, P.K.; Krishnamurthy, P. A Systematic phylogenomic classification of the multidrug and toxic compound extrusion transporter gene family in Plants. Front. Plant Sci. 2022, 13, 774885.
- Upadhyay, N.; Kar, D.; Deepak Mahajan, B.; Nanda, S.; Rahiman, R.; Panchakshari, N.; Bhagavatula, L.; Datta, S. The multitasking abilities of MATE transporters in plants. J. Exp. Bot. 2019, 70, 4643–4656.
- Liu, J.; Li, Y.; Wang, W.; Gai, J.; Li, Y. Genome-wide analysis of MATE transporters and expression patterns of a subgroup of MATE genes in response to aluminum toxicity in soybean. BMC Genom. 2016, 17, 223.
- Shitan, N.; Minami, S.; Morita, M.; Hayashida, M.; Ito, S.; Takanashi, K.; Omote, H.; Moriyama, Y.; Sugiyama, A.; Goossens, A.; et al. Involvement of the Leaf-Specific Multidrug and Toxic Compound Extrusion (MATE) Transporter Nt-JAT2 in Vacuolar Sequestration of Nicotine in Nicotiana tabacum. PLoS ONE 2014, 9, e108789.
- Morita, M.; Shitan, N.; Sawada, K.; Van Montagu, M.C.; Inzé, D.; Rischer, H.; Goossens, A.; Oksman-Caldentey, K.M.; Moriyama, Y.; Yazaki, K. Vacuolar transport of nicotine is mediated by a multidrug and toxic compound extrusion (MATE) transporter in Nicotiana tabacum. Proc. Natl. Acad. Sci. USA 2009, 106, 2447–2452.
- Tsay, Y.-F.; Schroeder, J.I.; Feldmann, K.A.; Crawford, N.M. The herbicide sensitivity gene CHL1 of Arabidopsis encodes a nitrate-inducible nitrate transporter. Cell 1993, 72, 705–713.
- Paulsen, I.T.; Skurray, R.A. The POT family of transport proteins. Trends Biochem. Sci. 1994, 19, 404.
- Steiner, H.-Y.; Naider, F.; Becker, J.M. The PTR family: A new group of peptide transporters. Mol. Microbiol. 1995, 16, 825–834.
- Sun, J.; Bankston, J.R.; Payandeh, J.; Hinds, T.R.; Zagotta, W.N.; Zheng, N. Crystal structure of the plant dual-affinity nitrate transporter NRT1.1. Nature 2014, 507, 73–77.
- Corratgé-Faillie, C.; Lacombe, B. Substrate (un)specificity of Arabidopsis NRT1/PTR FAMILY (NPF) proteins. J. Exp. Bot. 2017, 68, 3107–3113.
- Longo, A.; Miles, N.W.; Dickstein, R. Genome Mining of Plant NPFs reveals varying conservation of signature motifs associated with the mechanism of transport. Front. Plant Sci. 2018, 9, 1668.
- Payne, R.M.E.; Xu, D.; Foureau, E.; Teto Carqueijeiro, M.I.S.; Oudin, A.; Bernonville, T.D.d.; Novak, V.; Burow, M.; Olsen, C.-E.; Jones, D.M.; et al. An NPF transporter exports a central monoterpene indole alkaloid intermediate from the vacuole. Nat. Plants 2017, 3, 16208.
- de Brito Francisco, R.; Martinoia, E. The vacuolar transportome of plant specialized metabolites. Plant Cell Physiol. 2018, 59, 1326–1336.
- Kazachkova, Y.; Zemach, I.; Panda, S.; Bocobza, S.; Vainer, A.; Rogachev, I.; Dong, Y.; Ben-Dor, S.; Veres, D.; Kanstrup, C.; et al. The GORKY glycoalkaloid transporter is indispensable for preventing tomato bitterness. Nat. Plants 2021, 7, 468–480.
- Léran, S.; Varala, K.; Boyer, J.-C.; Chiurazzi, M.; Crawford, N.; Daniel-Vedele, F.; David, L.; Dickstein, R.; Fernandez, E.; Forde, B.; et al. A unified nomenclature of NITRATE TRANSPORTER 1/PEPTIDE TRANSPORTER family members in plants. Trends Plant Sci. 2014, 19, 5–9.
- Hildreth, S.B.; Gehman, E.A.; Yang, H.; Lu, R.H.; Ritesh, K.C.; Harich, K.C.; Yu, S.; Lin, J.; Sandoe, J.L.; Okumoto, S.; et al. Tobacco nicotine uptake permease (NUP1) affects alkaloid metabolism. Proc. Natl. Acad. Sci. USA 2011, 108, 18179–18184.
- Kato, K.; Shitan, N.; Shoji, T.; Hashimoto, T. Tobacco NUP1 transports both tobacco alkaloids and vitamin B6. Phytochemistry 2015, 113, 33–40.
- Dastmalchi, M.; Chang, L.; Chen, R.; Yu, L.; Chen, X.; Hagel, J.M.; Facchini, P.J. Purine Permease-Type Benzylisoquinoline Alkaloid Transporters in Opium Poppy. Plant Physiol. 2019, 181, 916–933.
- Jelesko, J.G. An expanding role for purine uptake permease-like transporters in plant secondary metabolism. Front. Plant Sci. 2012, 3, 78.
- Chen, H.; Lan, H.; Huang, P.; Zhang, Y.; Yuan, X.; Huang, X.; Huang, J.; Zhang, H. Characterization of OsPM19L1 encoding an AWPM-19-like family protein that is dramatically induced by osmotic stress in rice. Genet. Mol. Res. 2015, 14, 11994–12005.
- Alexander, R.D.; Castillejo-Pons, P.; Alsaif, O.; Stahl, Y.; Seale, M.; Morris, P.C. The conserved plant PM19 protein functions as an osmosensor and regulator of germination. bioRxiv 2020.
- Chen, H.Y.; Huh, J.H.; Yu, Y.C.; Ho, L.H.; Chen, L.Q.; Tholl, D.; Frommer, W.B.; Guo, W.J. The Arabidopsis vacuolar sugar transporter SWEET2 limits carbon sequestration from roots and restricts Pythium infection. Plant J. 2015, 83, 1046–1058.
- Breia, R.; Conde, A.; Badim, H.; Fortes, A.M.; Gerós, H.; Granell, A. Plant SWEETs: From sugar transport to plant-pathogen interaction and more unexpected physiological roles. Plant Physiol. 2021, 186, 836–852.
- Ji, J.; Yang, L.; Fang, Z.; Zhang, Y.; Zhuang, M.; Lv, H.; Wang, Y. Plant SWEET Family of Sugar Transporters: Structure, Evolution and Biological Functions. Biomolecules 2022, 12, 205.
- Eom, J.S.; Chen, L.Q.; Sosso, D.; Julius, B.T.; Lin, I.W.; Qu, X.Q.; Braun, D.M.; Frommer, W.B. SWEETs, transporters for intracellular and intercellular sugar translocation. Curr. Opin. Plant Biol. 2015, 25, 53–62.
- Křeček, P.; Skůpa, P.; Libus, J.; Naramoto, S.; Tejos, R.; Friml, J.; Zažímalová, E. The PIN-FORMED (PIN) protein family of auxin transporters. Genome Biol. 2009, 10, 249.
- Zhou, J.-J.; Luo, J. The PIN-FORMED Auxin Efflux Carriers in Plants. Int. J. Mol. Sci. 2018, 19, 2759.
- Sauer, M.; Kleine-Vehn, J. PIN-FORMED and PIN-LIKES auxin transport facilitators. Development 2019, 146, dev168088.
- Xin, A.; Herburger, K. Mini review: Transport of hydrophobic polymers into the plant apoplast. Front. Plant Sci. 2021, 11, 590990.
- Edqvist, J.; Blomqvist, K.; Nieuwland, J.; Salminen, T.A. Plant lipid transfer proteins: Are we finally closing in on the roles of these enigmatic proteins? J. Lipid Res. 2018, 59, 1374–1382.
- Salminen, T.A.; Eklund, D.M.; Joly, V.; Blomqvist, K.; Matton, D.P.; Edqvist, J. Deciphering the evolution and development of the cuticle by studying lipid transfer proteins in mosses and liverworts. Plants 2018, 7, 6.
- Yokoyama, H.; Yokoyama, T.; Yuasa, M.; Fujimoto, H.; Sakudoh, T.; Honda, N.; Fugo, H.; Tsuchida, K. Lipid transfer particle from the silkworm, Bombyx mori, is a novel member of the apoB/large lipid transfer protein family. J. Lipid Res. 2013, 54, 2379–2390.
- Bandara, S.; Ramkumar, S.; Imanishi, S.; Thomas, L.D.; Sawant, O.B.; Imanishi, Y.; von Lintig, J. Aster proteins mediate carotenoid transport in mammalian cells. Proc. Natl. Acad. Sci. USA 2022, 119, e2200068119.
- Huang, M.D.; Chen, T.L.; Huang, A.H. Abundant type III lipid transfer proteins in Arabidopsis tapetum are secreted to the locule and become a constituent of the pollen exine. Plant Physiol. 2013, 163, 1218–1229.
- Aharoni, A.; Jongsma, M.A.; Kim, T.K.; Ri, M.B.; Giri, A.P.; Verstappen, F.W.A.; Schwab, W.; Bouwmeester, H.J. Metabolic Engineering of Terpenoid Biosynthesis in Plants. Phytochem. Rev. 2006, 5, 49–58.
- Pichersky, E.; Gershenzon, J. The formation and function of plant volatiles: Perfumes for pollinator attraction and defense. Curr. Opin. Plant Biol. 2002, 5, 237–243.
- Trapp, S.C.; Croteau, R.B. Genomic organization of plant terpene synthases and molecular evolutionary implications. Genetics 2001, 158, 811–832.
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in Pharmacological Activities of Terpenoids. Nat. Prod. Commun. 2020, 15, 1934578X20903555.
- Cox-Georgian, D.; Ramadoss, N.; Dona, C.; Basu, C. Therapeutic and medicinal uses of terpenes. Med. Plants Farm Pharm. 2019, 333–359.
- Touaibia, M.; Boutekedjiret, C.; Perino, S.; Chemat, F. Natural Terpenes as Building Blocks for Green Chemistry. In Plant Based “Green Chemistry 2.0”: Moving from Evolutionary to Revolutionary; Springer: Berlin/Heidelberg, Germany, 2019; pp. 171–195.
- Nagegowda, D.A. Plant volatile terpenoid metabolism: Biosynthetic genes, transcriptional regulation and subcellular compartmentation. FEBS Lett. 2010, 584, 2965–2973.
- Xiao, H.; Zhang, Y.; Wang, M. Discovery and engineering of cytochrome P450s for terpenoid biosynthesis. Trends Biotechnol. 2019, 37, 618–631.
- Jaramillo-Madrid, A.C.; Lacchini, E.; Goossens, A. Within and beyond organelle engineering: Strategies for increased terpene production in yeasts and plants. Curr. Opin. Green Sustain. Chem. 2022, 33, 100572.
- Nagegowda, D.A.; Gupta, P. Advances in biosynthesis, regulation, and metabolic engineering of plant specialized terpenoids. Plant Sci. 2020, 294, 110457.
- Demurtas, O.C.; Frusciante, S.; Ferrante, P.; Diretto, G.; Azad, N.H.; Pietrella, M.; Aprea, G.; Taddei, A.R.; Romano, E.; Mi, J.; et al. Candidate enzymes for saffron crocin biosynthesis are localized in multiple cellular compartments. Plant Physiol. 2018, 177, 990–1006.
- Binenbaum, J.; Weinstain, R.; Shani, E. Gibberellin Localization and Transport in Plants. Trends Plant Sci. 2018, 23, 410–421.
- Feng, Z.; Bartholomew, E.S.; Liu, Z.; Cui, Y.; Dong, Y.; Li, S.; Wu, H.; Ren, H.; Liu, X. Glandular trichomes: New focus on horticultural crops. Hortic. Res. 2021, 8, 158.
- Yazaki, K.; Arimura, G.-i.; Ohnishi, T. ‘Hidden’ terpenoids in plants: Their biosynthesis, localization and ecological roles. Plant Cell Physiol. 2017, 58, 1615–1621.
- Fu, X.; Shi, P.; He, Q.; Shen, Q.; Tang, Y.; Pan, Q.; Ma, Y.; Yan, T.; Chen, M.; Hao, X.; et al. AaPDR3, a PDR Transporter 3, is involved in sesquiterpene β-caryophyllene transport in Artemisia annua. Front. Plant Sci. 2017, 8, 723.
- Degenhardt, J.; Gershenzon, J.; Baldwin, I.T.; Kessler, A. Attracting friends to feast on foes: Engineering terpene emission to make crop plants more attractive to herbivore enemies. Curr. Opin. Biotechnol. 2003, 14, 169–176.
- Chen, F.; Tholl, D.; D’Auria, J.C.; Farooq, A.; Pichersky, E.; Gershenzon, J. Biosynthesis and emission of terpenoid volatiles from Arabidopsis flowers. Plant Cell 2003, 15, 481–494.
- Kappers, I.F.; Aharoni, A.; van Herpen, T.W.; Luckerhoff, L.L.; Dicke, M.; Bouwmeester, H.J. Genetic engineering of terpenoid metabolism attracts bodyguards to Arabidopsis. Science 2005, 309, 2070–2072.
- Falleh, H.; Jemaa, M.B.; Saada, M.; Ksouri, R. Essential oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 127268.
- Assis, D.B.; Aragão Neto, H.C.; da Fonsêca, D.V.; de Andrade, H.H.N.; Braga, R.M.; Badr, N.; Maia, M.D.S.; Castro, R.D.; Scotti, L.; Scotti, M.T.; et al. Antinociceptive activity of chemical components of essential oils that involves docking studies: A review. Front. Pharmacol. 2020, 11, 777.
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. 2022, 13, 100217.
- Mohammed, A.E.; Abdul-Hameed, Z.H.; Alotaibi, M.O.; Bawakid, N.O.; Sobahi, T.R.; Abdel-Lateff, A.; Alarif, W.M. Chemical diversity and bioactivities of monoterpene indole alkaloids (MIAs) from six apocynaceae genera. Molecules 2021, 26, 488.
- Rosales, P.F.; Bordin, G.S.; Gower, A.E.; Moura, S. Indole alkaloids: 2012 until now, highlighting the new chemical structures and biological activities. Fitoterapia 2020, 143, 104558.
- Legault, J.; Pichette, A. Potentiating effect of β-caryophyllene on anticancer activity of α-humulene, isocaryophyllene and paclitaxel. J. Pharm. Pharmacol. 2007, 59, 1643–1647.
- Huang, M.; Sanchez-Moreiras, A.M.; Abel, C.; Sohrabi, R.; Lee, S.; Gershenzon, J.; Tholl, D. The major volatile organic compound emitted from Arabidopsis thaliana flowers, the sesquiterpene (E)-β-caryophyllene, is a defense against a bacterial pathogen. New Phytol. 2012, 193, 997–1008.
- Xu, R.; Fazio, G.C.; Matsuda, S.P. On the origins of triterpenoid skeletal diversity. Phytochemistry 2004, 65, 261–291.
- Kumar, P.; Bhadauria, A.S.; Singh, A.K.; Saha, S. Betulinic acid as apoptosis activator: Molecular mechanisms, mathematical modeling and chemical modifications. Life Sci. 2018, 209, 24–33.
- Hayashi, H.; Huang, P.; Kirakosyan, A.; Inoue, K.; Hiraoka, N.; Ikeshiro, Y.; Kushiro, T.; Shibuya, M.; Ebizuka, Y. Cloning and characterization of a cDNA encoding beta-amyrin synthase involved in glycyrrhizin and soyasaponin biosyntheses in licorice. Biol. Pharm. Bull. 2001, 24, 912–916.
- Sun, T.; Yuan, H.; Cao, H.; Yazdani, M.; Tadmor, Y.; Li, L. Carotenoid metabolism in plants: The role of plastids. Mol. Plant 2018, 11, 58–74.
- Simkin, A.J. Carotenoids and apocarotenoids in planta: Their role in plant development, contribution to the flavour and aroma of fruits and flowers, and their nutraceutical benefits. Plants 2021, 10, 2321.
- Felemban, A.; Braguy, J.; Zurbriggen, M.D.; Al-Babili, S. Apocarotenoids involved in plant development and stress response. Front. Plant Sci. 2019, 10, 1168.
- Moreno, J.C.; Mi, J.; Alagoz, Y.; Al-Babili, S. Plant apocarotenoids: From retrograde signaling to interspecific communication. Plant J. 2021, 105, 351–375.
- Burla, B.; Pfrunder, S.; Nagy, R.; Francisco, R.M.; Lee, Y.; Martinoia, E. Vacuolar Transport of Abscisic Acid Glucosyl Ester Is Mediated by ATP-Binding Cassette and Proton-Antiport Mechanisms in Arabidopsis. Plant Physiol. 2013, 163, 1446–1458.
- Demurtas, O.C.; de Brito Francisco, R.; Diretto, G.; Ferrante, P.; Frusciante, S.; Pietrella, M.; Aprea, G.; Borghi, L.; Feeney, M.; Frigerio, L.; et al. ABCC transporters mediate the vacuolar accumulation of crocins in saffron stigmas. Plant Cell 2019, 31, 2789–2804.
- Caissard, J.C.; Joly, C.; Bergougnoux, V.; Hugueney, P.; Mauriat, M.; Baudino, S. Secretion mechanisms of volatile organic compounds in specialized cells of aromatic plants. Recent Res. Dev. Cell Biol. 2004, 2, 1–15.
- Effmert, U.; Große, J.; Röse, U.S.; Ehrig, F.; Kägi, R.; Piechulla, B. Volatile composition, emission pattern, and localization of floral scent emission in Mirabilis jalapa (Nyctaginaceae). Am. J. Bot. 2005, 92, 2–12.
- Niinemets, Ü.; Loreto, F.; Reichstein, M. Physiological and physicochemical controls on foliar volatile organic compound emissions. Trends Plant Sci. 2004, 9, 180–186.
- Ichino, T.; Yazaki, K. Modes of secretion of plant lipophilic metabolites via ABCG transporter-dependent transport and vesicle-mediated trafficking. Curr. Opin. Plant Biol. 2022, 66, 102184.
- Gomez, C.; Conejero, G.; Torregrosa, L.; Cheynier, V.; Terrier, N.; Ageorges, A. In vivo grapevine anthocyanin transport involves vesicle-mediated trafficking and the contribution of anthoMATE transporters and GST. Plant J. 2011, 67, 960–970.
- Poustka, F.; Irani, N.G.; Feller, A.; Lu, Y.; Pourcel, L.; Frame, K.; Grotewold, E. A trafficking pathway for anthocyanins overlaps with the endoplasmic reticulum-to-vacuole protein-sorting route in Arabidopsis and contributes to the formation of vacuolar inclusions. Plant Physiol. 2007, 145, 1323–1335.
- Schwab, W.; Fischer, T.; Wüst, M. Terpene glucoside production: Improved biocatalytic processes using glycosyltransferases. Eng. Life Sci. 2015, 15, 376–386.
- Aharoni, A.; Giri, A.P.; Deuerlein, S.; Griepink, F.; de Kogel, W.J.; Verstappen, F.W.A.; Verhoeven, H.A.; Jongsma, M.A.; Schwab, W.; Bouwmeester, H.J. Terpenoid metabolism in wild-type and transgenic Arabidopsis plants. Plant Cell 2003, 15, 2866–2884.
- Lücker, J.; Bowen, P.; Bohlmann, J. Vitis vinifera terpenoid cyclases: Functional identification of two sesquiterpene synthase cDNAs encoding (+)-valencene synthase and (−)-germacrene D synthase and expression of mono-and sesquiterpene synthases in grapevine flowers and berries. Phytochemistry 2004, 65, 2649–2659.
- Ohara, K.; Ujihara, T.; Endo, T.; Sato, F.; Yazaki, K. Limonene production in tobacco with Perilla limonene synthase cDNA. J. Exp. Bot. 2003, 54, 2635–2642.
- Adebesin, F.; Widhalm, J.R.; Boachon, B.; Lefèvre, F.; Pierman, B.; Lynch, J.H.; Alam, I.; Junqueira, B.; Benke, R.; Ray, S.; et al. Emission of volatile organic compounds from petunia flowers is facilitated by an ABC transporter. Science 2017, 356, 1386–1388.
- Martin, D.M.; Toub, O.; Chiang, A.; Lo, B.C.; Ohse, S.; Lund, S.T.; Bohlmann, J. The bouquet of grapevine (Vitis vinifera L. cv. Cabernet Sauvignon) flowers arises from the biosynthesis of sesquiterpene volatiles in pollen grains. Proc. Natl. Acad. Sci. USA 2009, 106, 7245–7250.
- Gersbach, P. The essential oil secretory structures of Prostanthera ovalifolia (Lamiaceae). Ann. Bot. 2002, 89, 255–260.
- Skubatz, H.; Kunkel, D.D.; Patt, J.M.; Howald, W.N.; Hartman, T.G.; Meeuse, B. Pathway of terpene excretion by the appendix of Sauromatum guttatum. Proc. Natl. Acad. Sci. USA 1995, 92, 10084–10088.
- Park, K.-R.; Nam, D.; Yun, H.-M.; Lee, S.-G.; Jang, H.-J.; Sethi, G.; Cho, S.K.; Ahn, K.S. β-Caryophyllene oxide inhibits growth and induces apoptosis through the suppression of PI3K/AKT/mTOR/S6K1 pathways and ROS-mediated MAPKs activation. Cancer Lett. 2011, 312, 178–188.
- Shitan, N.; Yazaki, K. Dynamism of vacuoles toward survival strategy in plants. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183127.
- Jasiński, M.; Stukkens, Y.; Degand, H.; Purnelle, B.; Marchand-Brynaert, J.; Boutry, M. A Plant Plasma Membrane ATP Binding Cassette–Type Transporter Is Involved in Antifungal Terpenoid Secretion. Plant Cell 2001, 13, 1095–1107.
- Stukkens, Y.; Bultreys, A.; Grec, S.; Trombik, T.; Vanham, D.; Boutry, M. NpPDR1, a pleiotropic drug resistance-type ATP-binding cassette transporter from Nicotiana plumbaginifolia, plays a major role in plant pathogen defense. Plant Physiol. 2005, 139, 341–352.
- Crouzet, J.; Roland, J.; Peeters, E.; Trombik, T.; Ducos, E.; Nader, J.; Boutry, M. NtPDR1, a plasma membrane ABC transporter from Nicotiana tabacum, is involved in diterpene transport. Plant Mol. Biol. 2013, 82, 181–192.
- Pierman, B.; Toussaint, F.; Bertin, A.; Lévy, D.; Smargiasso, N.; De Pauw, E.; Boutry, M. Activity of the purified plant ABC transporter NtPDR1 is stimulated by diterpenes and sesquiterpenes involved in constitutive and induced defenses. J. Biol. Chem. 2017, 292, 19491–19502.
- Shibata, Y.; Ojika, M.; Sugiyama, A.; Yazaki, K.; Jones, D.A.; Kawakita, K.; Takemoto, D. The full-size ABCG transporters Nb-ABCG1 and Nb-ABCG2 function in pre-and postinvasion defense against Phytophthora infestans in Nicotiana benthamiana. Plant Cell 2016, 28, 1163–1181.
- Rin, S.; Mizuno, Y.; Shibata, Y.; Fushimi, M.; Katou, S.; Sato, I.; Chiba, S.; Kawakita, K.; Takemoto, D. EIN2-mediated signaling is involved in pre-invasion defense in Nicotiana benthamiana against potato late blight pathogen, Phytophthora infestans. Plant Signal. Behav. 2017, 12, e1300733.
- Campbell, E.J.; Schenk, P.M.; Kazan, K.; Penninckx, I.A.; Anderson, J.P.; Maclean, D.J.; Cammue, B.P.; Ebert, P.R.; Manners, J.M. Pathogen-responsive expression of a putative ATP-binding cassette transporter gene conferring resistance to the diterpenoid sclareol is regulated by multiple defense signaling pathways in Arabidopsis. Plant Physiol. 2003, 133, 1272–1284.
- Van Den Brûle, S.; Müller, A.; Fleming, A.J.; Smart, C.C. The ABC transporter SpTUR2 confers resistance to the antifungal diterpene sclareol. Plant J. 2002, 30, 649–662.
- Smart, C.C.; Fleming, A.J. Hormonal and environmental regulation of a plant PDR5-like ABC transporter. J. Biol. Chem. 1996, 271, 19351–19357.
- Sasse, J.; Schlegel, M.; Borghi, L.; Ullrich, F.; Lee, M.; Liu, G.W.; Giner, J.L.; Kayser, O.; Bigler, L.; Martinoia, E.; et al. Petunia hybrida PDR2 is involved in herbivore defense by controlling steroidal contents in trichomes. Plant Cell Environ. 2016, 39, 2725–2739.
- Kumari, A.; Pandey, N.; Pandey-Rai, S. Exogenous salicylic acid-mediated modulation of arsenic stress tolerance with enhanced accumulation of secondary metabolites and improved size of glandular trichomes in Artemisia annua L. Protoplasma 2018, 255, 139–152.
- Wang, B.; Kashkooli, A.B.; Sallets, A.; Ting, H.-M.; de Ruijter, N.C.A.; Olofsson, L.; Brodelius, P.; Pottier, M.; Boutry, M.; Bouwmeester, H.; et al. Transient production of artemisinin in Nicotiana benthamiana is boosted by a specific lipid transfer protein from A. annua. Metab. Eng. 2016, 38, 159–169.
- Yan, L.; Zhang, J.; Chen, H.; Luo, H. Genome-wide analysis of ATP-binding cassette transporter provides insight to genes related to bioactive metabolite transportation in Salvia miltiorrhiza. BMC Genom. 2021, 22, 315.
- Kato, K.; Horiba, A.; Hayashi, H.; Mizukami, H.; Terasaka, K. Characterization of Triterpene Saponin Glycyrrhizin Transport by Glycyrrhiza glabra. Plants 2022, 11, 1250.
- Mylona, P.; Owatworakit, A.; Papadopoulou, K.; Jenner, H.; Qin, B.; Findlay, K.; Hill, L.; Qi, X.; Bakht, S.; Melton, R.; et al. Sad3 and sad4 are required for saponin biosynthesis and root development in oat. Plant Cell 2008, 20, 201–212.
- Yu, F.; De Luca, V. ATP-binding cassette transporter controls leaf surface secretion of anticancer drug components in Catharanthus roseus. Proc. Natl. Acad. Sci. USA 2013, 110, 15830–15835.
- Kramer, E.M. How Far Can a Molecule of Weak Acid Travel in the Apoplast or Xylem? Plant Physiol. 2006, 141, 1233–1236.
- Lacombe, B.; Achard, P. Long-distance transport of phytohormones through the plant vascular system. Curr. Opin. Plant Biol. 2016, 34, 1–8.
- Kanno, Y.; Hanada, A.; Chiba, Y.; Ichikawa, T.; Nakazawa, M.; Matsui, M.; Koshiba, T.; Kamiya, Y.; Seo, M. Identification of an abscisic acid transporter by functional screening using the receptor complex as a sensor. Proc. Natl. Acad. Sci. USA 2012, 109, 9653–9658.
- Chiba, Y.; Shimizu, T.; Miyakawa, S.; Kanno, Y.; Koshiba, T.; Kamiya, Y.; Seo, M. Identification of Arabidopsis thaliana NRT1/PTR FAMILY (NPF) proteins capable of transporting plant hormones. J. Plant Res. 2015, 128, 679–686.
- Tal, I.; Zhang, Y.; Jørgensen, M.E.; Pisanty, O.; Barbosa, I.C.; Zourelidou, M.; Regnault, T.; Crocoll, C.; Olsen, C.E.; Weinstain, R.; et al. The Arabidopsis NPF3 protein is a GA transporter. Nat. Commun. 2016, 7, 11486.
- Kanno, Y.; Oikawa, T.; Chiba, Y.; Ishimaru, Y.; Shimizu, T.; Sano, N.; Koshiba, T.; Kamiya, Y.; Ueda, M.; Seo, M. AtSWEET13 and AtSWEET14 regulate gibberellin-mediated physiological processes. Nat. Commun. 2016, 7, 13245.
- Morii, M.; Sugihara, A.; Takehara, S.; Kanno, Y.; Kawai, K.; Hobo, T.; Hattori, M.; Yoshimura, H.; Seo, M.; Ueguchi-Tanaka, M. The Dual Function of OsSWEET3a as a Gibberellin and Glucose Transporter Is Important for Young Shoot Development in Rice. Plant Cell Physiol. 2020, 61, 1935–1945.
- Shani, E.; Binenbaum, J.; Wulff, N.; Camut, L.; Kiradjiev, K.; Tal, I.; Vasuki, H.; Anfang, M.; Zhang, Y.; Sakvarelidze-Achard, L.; et al. Gibberellin and Abscisic Acid Transporters Facilitate Endodermal Suberin Formation in Arabidopsis. Preprint Version Available at Research Square. 2022. Available online: https://assets.researchsquare.com/files/rs-1670556/v1/2f32f876-23ab-4264-899c-99b89c8437f2.pdf?c=1653493635 (accessed on 10 October 2022).
More
