1. Interaction of Chromolaena odorata with the Natural Enemy
The interaction between the invasive plants and their natural enemies such as herbivores and pathogens, is one of the important factors for the naturalization of the invasive plants
[1][2][3][4][5]. The population of
C. odorata is controlled by many insects and pathogens in its native ranges
[6][7]. More than 200 species of the herbivores were counted in the native ranges of
C. odorata, and 25% of them are specific species in the native ranges
[8]. There may be fewer specific herbivores in the invasive ranges. In fact, very few specific insect species for
C. odorata in the invasive range (South Africa) were counted
[9]. According to the evolution of increased competitive ability hypothesis, the success of the invasive species is due to fewer specialized predators in the invasive ranges. The invasive plants can allocate the resources from the high-cost defense strategy to the low-cost defense strategy and plant growth, leading to the successful naturalization
[10][11].
2. Interaction of C. odorata with Insects
Powder of the roots, stems, and leaves of
C. odorata increased the mortality of leaf beetle,
Callosobruchus maculatus Fabricius
[12][13]. Essential oil obtained from
C. odorata leaves also increased the mortality of adult weevil,
Sitophilus zeamais Motschulsky
[14][15]. Aqueous leaf extract of
C. odorata induced the increasing larval mortality of black fly,
Simulium spp.
[16], and an adult stage of cockroach,
Periplaneta americana Linnaeus
[17]. Aqueous ethanol leaf extracts of
C. odorata were applied to
Aabelmoschus esculentus (L.) Moench once a week for 4–7 weeks after its planning. The treatments resulted in the reduction of the population of whitefly,
Bemissa tabaci Gennadius, and leafhopper,
Amrasca biguttula Ishida on
Aabelmoschus esculentus [18]. The methanol extracts of
C. odorata leaves also showed ovicidal, antifeedant, and larvicidal activity on a leaf-eating insect,
Spodoptera litura Fabricius
[19]. These observations suggest that
C. odorata possess anti-insect activity and contain certain compounds involved in the activity.
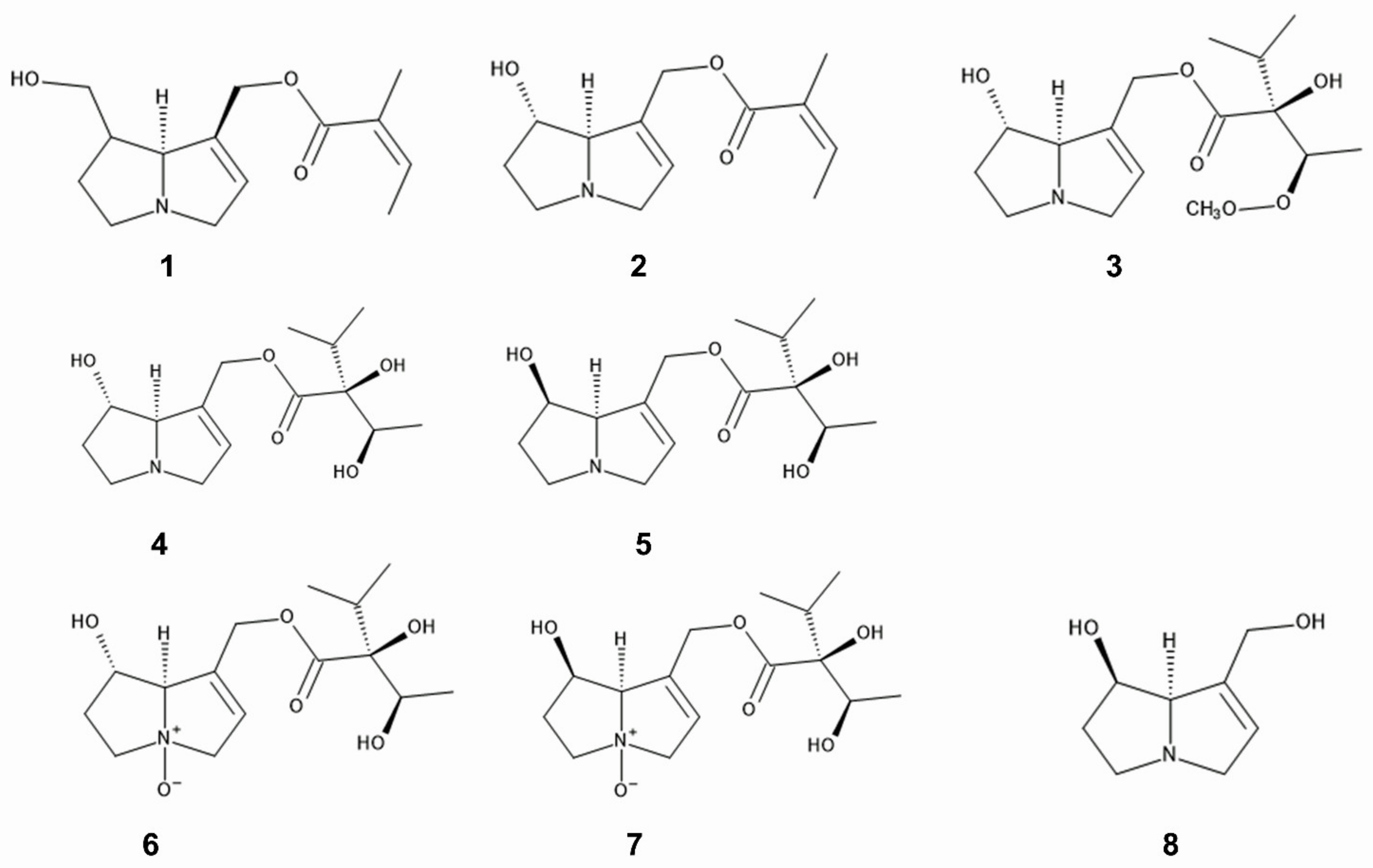
Pyrrolizidine alkaloids such as 7- and 9-angeloylretronecine, intermedine, rinderine, and 3′-acetylrinderine were isolated from roots and mature flower heads of
C. odorata [20], and rinderine
N-oxide and intermedine
N-oxide were identified in its roots
[21] (
Figure 21). Pyrrolizidine alkaloids are amino alcohols, esterified with mono- or dicarboxylic acids
[22], and act as chemical defense agents against herbivores such as insects and mammals
[23][24][25][26]. The compounds are highly toxic including hepatotoxicity, and disturb several metabolisms in the cell functions
[27][28][29][30]. Therefore, these pyrrolizidine alkaloids in
C. odorata may be involved in the anti-insect activity caused by the extracts and power of the species as describe above, and contribute to the protection of the species from herbivore attacks.
Figure 21. Pyrrolizidine alkaloids; 1: 7-angeloylretronecine, 2: 9-angeloylretronecine, 3: 3′-acetylrinderine, 4; rinderine, 5: intermedine, 6: rinderine N-oxide, 7: intermedine N-oxide, 8: 1,2-dehydropyrrolizine alkaloid.
Some specialist insects obtain pyrrolizidine alkaloids from plants and store them. Those stored pyrrolizidine alkaloids are used for their protection from their predators as poison, and for the precursors to synthesize their mail pheromones
[22][31][32]. However,
C. odorata may scarcely meet these specialist insects in its invasive ranges since the host plants of the sspecialist insects are narrow and there may be no such co-evolutional history between
C. odorata and the insects in the invasive ranges (
Figure 2,
Table 1).
Table 1. Interaction of C. odorata with insects.
3. Interaction of C. odorata with Nematodes
Plant parasitic nematodes such as
Meloidogyne spp. (root-knot nematode),
Helicotylenchus spp., and
Pratylenchus spp. feed on the roots of plants, and their feeding process causes serious injuries and reduces the ability of the plants to absorb nutrients and water, leading to losing plant vigor and defense capability against other pathogen attacks
[33][34][35]. The population density of nematodes such as
Meloidogyne spp.,
Helicotylenchus spp. and
Pratylenchus spp., was suppressed by the
C. odorata infestation into the invasive ranges with various soil conditions
[36]. It was also reported that
C. dorata reduced by 77–81% of plant parasitic nematode population;
Meloidogyne spp.,
Helicotylenchus spp., and
Pratylenchus spp. in the soils after two years invention
[37].
C. odorata also suppressed the increasing population of
Meloidogyne incoginita Kofoid & White in the pot experiments
[38]. These observations suggest that some compounds may be released from
C. odorata and accumulated in the soils, and these compounds may suppress the population of plant parasitic nematodes in the soils.
The incorporation of plant powder of
C. odorata into the field soil prevented the increasing population of
Meloidogyne incoginita [39]. Aqueous root extracts and root mulch of
C. odorata showed the suppression of the parasitism of
Meloidogyne incoginita into the roots of
Lactuca sativa L.
[40]. Therefore, certain compounds in the plant powder, roots, and extracts of
C. odorata may work for the suppression.
1,2-Dehydropyrrolizine alkaloid was identified in the root extracts of
C. odorata and the compound showed anti-nematode activity
[40] (
Figure 2). 1,2-Dehydropyrrolizine alkaloid was reported to be synthesized and stored in vacuole in the roots of
C. odorata [41]. Pyrrolizidine alkaloids are highly toxic and act as chemical defense agents against natural enemies
[23][24][25]. Those observations suggest that the extracts and powder
of C. odorata, and the soil under
C. odorata may suppress the population of the nematodes, and prevented the hatch and parasitism of the nematodes. Certain compounds including 1,2-dehydropyrrolizine alkaloid may cause the suppression and acts as anti-nematode agents of
C. odorata (
Table 2).
Table 2. Interaction of C. odorata with nematodes.
4. Interaction of C. odorata with Microbial
The invasion of
C. odorata into the forest and savanna in West Africa increased the soil microbial activity, and the amount of available N and P in the soil.
C. odorata altered the soil microbial community in the invasion ranges. The altered microbial community suppressed the growth of the native plant species such as
Eupatorium japonicum Thunb. and
Eupatorium heterophyllum DC., and stimulated the growth of
C. odorata [42]. The population of an arbuscular mycorrhizal fungus
Paraglomus spp. was also increased in the soil under
C. odorata [43]. Arbuscular mycorrhizal fungi increase the ability of their host plants to absorb water and nutrients, and enhance the defense function against several stress conditions and pathogen attacks
[44][45][46]. The observations suggest that certain compounds from
C. odorata may alter the soil microbial community in the invasion ranges and the alteration may contribute the invasion of
C. odorata. However, an arbuscular mycorrhizal fungus
Paraglomus spp. colonizes with a wide range of plant species
[47][48]. The colonization may occur with other plant species and promote their growth in the invasive ranges of
C. odorata. In addition, the abundance of the arbuscular mycorrhizal colonization of
C. odorata in its invasive range (South Africa) was reported to be 50% of its native ranges (Puerto Rico)
[49].
The rhizosphere soil of
C. odorata increased the population of the soil borne fungal pathogen,
Fusarium ssp., and inhibited the growth of
Amaranthus spinosus L. and
Bambusa bambos (L.) Voss. Sterilization of the soil eliminated these effects. The root leachate of
C. odorata increased the spore density of
Fusarium spp. in
C. odorata-free soil. The increases were illuminated by adding activated carbon into the soil
[50]. It was also reported that the root exudates of
Sorghum bicolor (L.) Moench. and
Vigna unguiculata (L.) Walp. increased the population of
Fusarium spp.
[51], and phenolics in the root exudates of
Glycine max (L.) Merr. increased the population of
Fusarium spp.
[52]. The observations suggest that certain compounds in the root exudate of these plant species including
C. odorata may stimulate the increasing population of
Fusarium spp. However, it is not clear if the increased
Fusarium spp. population affects the growth of
C. odorata.On the contrary, it was reported that the extracts of
C. odorata suppressed the growth of some soil borne fungal pathogens including
Fusarium spp. Aqueous methanol leaf extracts of
C. odorata significantly suppressed the colony growth of the pathogens,
Lasiodiplodia theobromae (Pat.) Griffon & Maubl. and
Lasiodiplodia pseudothobromae A.J.L. Phillips, A. Alves & Crous
[53]. Both
Lasiodiplodia spp. are members of the Botryosphaeriaceae family and cause leaf necrosis, canker, and dieback in many plant species
[54][55]. The methanol leaf extracts of
C. odorata suppressed the growth of
Bacillus subtilis Cohn, and
Bacillus cereus Frankland & Frankland
[56]. The ethanol plant extracts of
C. odorata suppressed the growth of soil borne pathogen fungi,
Phytophthora colocasiae Racib., and
Fusarium oxysporum Schlecht. emend. Snyder & Hansen
[57]. Acetone extracts of
C. odorata also suppressed the colony growth of the pathogen fungi,
Pythium ultimum Trow,
Rhizoctonia solani J.G. Kühn,
Fusarium oxysporium Schlecht. emend. Snyder & Hansen, and
Phytophthora nicotianae Breda de Haan
[58] and
Pyricularia oryzae Cavara
[59]. Essential oil of
C. odorata suppressed the growth of
Rhizoctonia solani J.G. Kühn,
Fusarium graminearum Schwabe,
Exserohilum turcicum (Pass.) K.J. Leonard & Suggs,
Botrytis cinereal Pers., and
Sclerotinia sclerotiorum (Lib.) de Bary
[60]. These observations suggest that the extracts of
C. odorata possess the anti-fungal activity, and may contain certain compounds involved in the activity.
Some compounds in the extracts and/or the rhizosphere soil of C. odorata may be involved in the alteration of the microbial community as the observations in those publications (Table 3). The identification of these compounds is also necessary. In addition, the observations described in this section are controversial that whether C. odorata increases the population of fungal pathogen such as Fusarium spp. or suppressed the population. More sophisticate investigations are necessary in the future to explain the interaction of C. odorata with the microbial population.
Table 3. Interaction of C. odorata with microbial population.