Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Kishu Ranjan and Version 3 by Jessie Wu.
Endocytosis is a mechanistic process, associated with internalization of the extracellular materials such as microbes, cellular components, nutrients, or macromolecules. Conventionally, eukaryotic cells use the endocytosis process for the absorption of molecules and secretion of signaling transmitters (hormones and cytokines) to maintain cellular homeostasis. Endocytosis machinery is a well-conserved physiological process in lower to higher organisms, which has been frequently acquired for cellular defense, immune responses, uptake, and energy metabolism.
- endocytosis
- pinocytosis
- diseases
- Cancer
1. Cancer
Endocytosis is a complex cellular event involved in homeostasis and communication to extracellular milieu through internalization of the plasma membrane along with its integral membrane proteins, immunoglobulins, receptors and their ligands, and nutrients. It is a crucial signaling event that plays a key role in cell cycle regulation, mitosis, and apoptosis. Endocytosis is a regulated signaling mechanism and plays a potential role in tumor suppressor pathways. It plays a critical role in signaling through endosomes and rescue degradation of signaling molecules involved in cancer signaling, thus it appears as a potential target in oncogenic pathways. Further, endocytosis is involved in activation of certain cancer receptors such as Epidermal growth factor receptor (EGFR), Transferrin receptor (TfR), and Notch receptor [1][101]. In addition, in human tumors, altered expression of various endocytic regulatory factors such as clathrin-hc, clathrin- like, Caveolin-1, Nexin-1, and Numb along with driver mutations are crucial for endocytosis [2][3][102,103]. Endocytic protein Numb governs the level and activity of tumor suppressive protein p53. Numb inhibits the degradation of p53 by forming the tricomplex of p53 and Hdm2 where it suppresses the ligase activity of Hdm2 [4][104]. Thus, perturbation in Numb levels may alter the expression of the p53-associated cellular process, such as response to DNA damage, induction of checkpoint, and apoptosis-associated proteins [5][105]. Furthermore, a remarkable decline in the levels of Numb expression has been observed in approximately 50% of breast cancer [4][104]. The disruption of Numb expression might have a severe impact on tumorigenesis. The endocytic activity of Numb is associated to Numb–Notch interactions for cell proliferation and differentiation. Importantly, endocytosis plays an indispensable role in the aggressive nature of cancer. Endocytosis appears as an important regulator of tumor metastasis [6][106]. In cancer, deregulation of several endocytic proteins is involved in migration and invasion. There are some metastatic suppressor genes such as Kisspeptin-1 (KISS1), and metastasis suppressor protein 1 (MTSS1) whose activity depends on alteration in the endocytosis process [6][106]. Kisspeptin-1 (KISS-1) inhibits cell motility, proliferation, invasion, and metastasis in cancers [7][107]. However, in breast cancer, it induces invasion. MTSS1 acts as a scaffold protein and inhibits the metastasis in various cancers. However, in head and neck squamous cell carcinoma, a low level of MTSS1 augment the EGF signaling and induce cell proliferation [6][106]. In contrast, a high level of MTSS1 exhibits a negative influence on EGF signaling and triggers metastasis [8][108].
In addition, several studies showed the involvement of different classes of endocytic proteins in the invasion of multiple cancer types, including colon, breast, colorectal, and non-small cell lung carcinoma (NSCLC). Caveolin endocytic protein shows a regulatory function for breast, prostate, and ovarian cancer. In the early stage of these cancers, caveolin acts as a tumor suppressor, while in advanced stage, it is associated with tumor progression and metastasis [9][10][109,110]. Clathrin-mediated endocytic protein AP2 modulates the cell migration and invasion of pancreatic, ovarian, and melanoma cancer through CXCR2 [11][111]. Endosomal trafficking proteins such as ARF1 regulate breast cancer cell proliferation and migration through regulating the interaction of β1 integrin and protein of focal adhesion (paxillin, Fak, talin) [12][13][112,113]. ARF6 promotes cellular motility and invasion of glioma and breast cancer cells by inducing internalization of E-cadherin and breakdown of adherence junction [14][114]. Further, endocytic proteins of the RAB subfamily such as RAB3C and RAB3D control invasion and metastasis of colorectal and breast cancer, respectively. Elevated expression of RAB3C promotes in vivo migration, invasion, and metastasis of colorectal cancer while RAB3D induced breast cancer cell invasion by activating Akt/GSK-3β/Snail pathway [15][16][115,116]. Endosomal-associated protein RAB5 promotes tumor cell migration and invasion, focal adhesion turnover, and integrin trafficking of cancer cells [17][18][117,118]. RAB21 controls integrin-mediated cell adhesion and motility of cervical cancer cells. Earlier studies paved a way to identify autophagy signaling proteins as a target to map their interaction with endocytic proteins and cross-regulation in tumor progression. However, identifying such targets is still challenging. Targeting the most viable endocytosis-associated gene(s) may help to achieve this goal. One of the recent studies used RNAseq expression in Rubcn knockout (KO) and wildtype (WT) group (GSE118019) [19][119]. RWesearchers also analyzed Rubcn gene expression in two different conditions to further explore the list of gene signatures, pathways enriched in the presence as well as absence of Rubcn. In order to understand the phagocytosis in tumors and their consequences, reswearhcers performed gene set enrichment analysis (GSEA) (Figure 1). Interestingly, in GSEA, rwesearchers identified several important pathways such as apoptosis, glycolysis, hypoxia, and IFNγ response enriched in KO group (Figure 2). Similarly, using differential gene expression analysis, reswearchers identified several important genes such as Rab4a, Gzme, Glrp1 that were upregulated in Rubcn KO group. This analysis suggests that the knock-out of endocytosis-associated genes also accelerates a more immunogenic microenvironment.
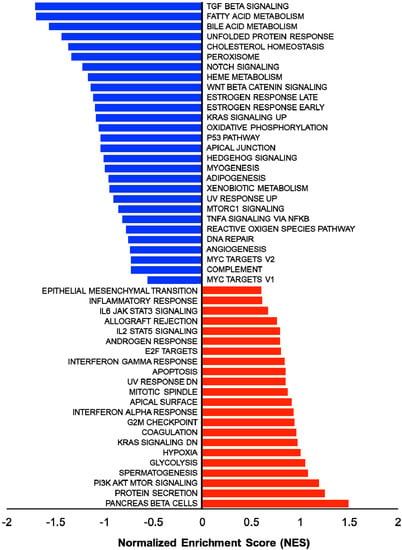
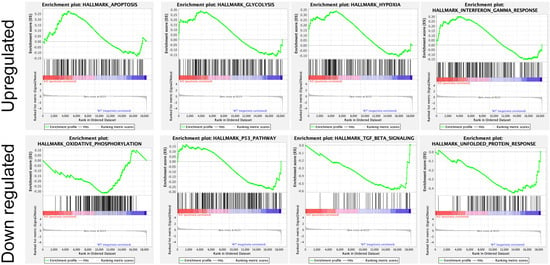
Figure 2. Enrichment plot of top four GSEA hallmarks enriched in Rubcn knockout and wildtype. Rubcn KO group enriched with significant upregulation in apoptosis, glycolysis, hypoxia, and interferon gamma response gene sets (upper panel). Oxidative phosphorylation, p53, TGFβ signaling, and unfolded protein response enriched in WT group (lower panel).
Cancer cells are hyper-proliferative and metastasize to the different part of the body. As a result of malignant proliferation, tumor cells need a rapid supply of nutrients for sustained proliferation. Macropinocytosis is a ligand-receptor-independent process and is exploited by cancer cells for rapid nutrient acquisition [20][120]. An earlier study showed that pancreatic cancer cell line KRPC was able to proliferate in the absence of essential amino acids in the culture media but obtain amino acids through macropinocytosis from extracellularly degraded albumin protein [21][121]. Macropinocytosis pathway can also facilitate the internalization of essential molecules, such as ATP, to mediate cancer cell proliferation and survival [22][122]. Macropinocytosis is also involved in K-Ras-mTORC1 signaling and may induce sustained mTORC1 activation and cell proliferation in cancer cells [23][123]. Moreover, macropinocytosis has been also reported in cell death of glioblastoma cancer cells with constitutive H-Ras activation in glioblastoma cell line U251, resulting in the accumulation macropinosomes and vacuolization of the cells [24][124].
2. Cardiovascular Disease
Cardiovascular diseases such as hypertension, coronary heart disease, stroke, and heart failure are the leading causes of mortality and morbidity [25][125]. Several endocytic proteins including sorting nexin (SNX), epsins, and disabled homolog 2 (Dab2) play an indispensable role in cardiovascular diseases [26][126]. SNX is a group of cytoplasmic and membrane-associated phosphoinositide binding proteins that play a role in protein trafficking [27][28][127,128]. Impairment of SNX pathway is responsible for the development of various forms of cardiovascular disease (CVD) [26][126]. In addition, SNX gene variants are also linked to CVD. Accumulating reports revealed that SNX exhibits its function by regulating expression and function of G protein-coupled receptors (GPCRs) such as receptor tyrosine kinases (RTKs) and dopamine receptors for the maintenance of blood pressure [29][30][129,130]. A previous report demonstrates that an impairment in the structure and function of SNX is associated with hypertension. Renal SNX5 expression regulates insulin degradation enzyme (IDE) activity and is associated with blood insulin and glucose levels [31][131]. Decreased renal expression of SNX5 expression leads to further elevation of systolic blood pressure and inhibition of sodium excretion [29][129]. Further, studies have also established the association of other SNX, such as SNX19, with coronary artery disease. However, the mechanistic pathways behind the SNXs-induced coronary artery disease remain enigmatic [26][126]. Moreover, SNXs may play a key role in coronary artery pathogenesis by regulating lipid metabolism. The influence of SNXs on a lipid level may be because of the interaction of SNX1, SNX2, and SNX4 with leptin receptors [32][132]. Furthermore, studies also suggested the abnormal expression of SNX leads to heart failure. Endogenous SNX13 level reduced in failing the heart of mice and human. In SNX13-deficient zebra fish, decreased cardiac systolic function was associated with cardiomyocyte apoptosis, and an inhibition of which improves the cardiac dysfunction [33][133]. In cardiovascular disease, atherosclerosis is a crucial player associated to morbidity and mortality. Epsins are endocytic adaptor proteins associated with cardiovascular disease [34][134]. Epsins are involved in endothelial cell dysfunction as well initiation and progression of arthrosclerosis through interaction with inositol 1,4,5 triphosphate receptor type 1 (IP3R1) [34][134]. Furthermore, Dab2, a multifactorial protein, plays a vital role in several cellular functions, including cell adhesion, cell signaling, and endocytosis. More importantly, Dab2 is associated with cholesterol metabolism and low-density lipoprotein (LDL) uptake by regulating the LDL receptor endocytosis. An earlier study suggests that deletion of Dab2 in liver endothelial cells results in an elevated level of serum LDL and cholesterol [35][135]. An earlier study shown that the Dab2 gene variant is associated with increased risk of coronary artery disease [36][136]. Interestingly, another report showed that quercetin-mediated up-regulation of Dab2 expression attenuates the atherosclerosis [37][137]. Hence, Dab2 is considered as a new anti-atherosclerosis therapeutic.
Earlier reports suggested that the CD36 (cluster of differentiation 36), a transmembrane glycoprotein receptor, plays an important role in athero-thrombotic activity and promotes the pathological conditions such as atherosclerosis and thrombosis [38][138]. CD36 is a pattern recognition receptor (PRR) and multi-functional protein that is majorly involved in the uptake of fatty acids (FA) in adipose tissues and plays a key role in regulation of lipid metabolism [39][139]. The process of FA uptake and its delivery is facilitated by caveolae-dependent internalization of CD36. Additionally, the FA uptake mediated by CD36 is a palmitoylation-regulated endocytic pathway [40][140]. The CD36 is expressed on the surface of various cell types including skeletal and cardiac myocytes [41][141]. It acts as a key player in energetics of cardiac myocytes as it facilitates FA transport, which further utilizes beta oxidation and leads to energy generation. Apart from FA, CD36 also recognizes and interacts with oxidized LDL (oxLDL), which eventually progresses atherosclerosis [42][142]. The oxLDL has been shown to promote apoptosis signaling in the vascular smooth muscle cells and contributes to atherosclerotic plaques [43][143]. The expression of CD36 on macrophages and platelets also promotes signaling cascades of inflammation which eventually participates in atherosclerotic arterial lesion formation and thrombus formation [44][144]. Collectively, CD36 dysfunction has been shown to contribute to the pathologies of atherosclerosis. Therefore, targeting the CD36-mediated transport of lipid moieties could be an effective therapeutic approach for the treatment of atherosclerosis and thrombosis.
Moreover, the intracellular compartmentalization of G protein-coupled receptor (GPCR) during early endosome and Golgi apparatus distribution are associated with cardiovascular outcomes. Endosomal G protein signaling by vasopressin type 2 receptor (V2R) plays a key role in cardiac arrest. There are three types of vasopressin receptors, including V1AR, V1BR, and V2R, which are triggered by arginine vasopressin (AVP). An elevated level of AVP plays a crucial role in changing the cardiovascular function and impaired renal solute-free water excretion result in hyponatremia [45][46][145,146]. Therefore, vasopressin receptors, V2R, have emerged as a popular target to develop the antagonist against therapeutics for cardiac arrest and hyponatremia.
3. Neurological Disorders
Millions of neurons organize and perform the regular functioning of the brain and nervous system. The fundamental role of neurons includes the transmission and receiving of the information or signals and behaving as a unified structure. This communication of information among the neurons is possible due to the presence of junction-like structures, known as synapses. The communication among neurons occurs by electrical or chemical signals. The electrical signal relies on the phenomena known as action potential. The chemical signals are generated through the transmission of various chemicals among neurons. Broadly, these chemicals are defined as neurotransmitters. The neurotransmitters are stored inside the vesicle structures and tend to release at the synaptic cleft of the synapse for the transmission of the information [47][147]. At the synapse, the release of neurotransmitters relies on the two fundamental biological events, such as exocytosis and endocytosis. The process of exocytosis is responsible for the release of neurotransmitters, while the process of endocytosis is responsible for the recycling of the synaptic vesicle membranes [48][148].
Several events occur during the process of chemical neurotransmission, such as formation of synaptic vesicles (SV), fusion with plasma membrane for releasing neurotransmitters, and recycling of synaptic vesicles. The recycling of synaptic vesicles is the hallmark event which comprises three key steps at the synapse; (i) release of neurotransmitters, (ii) clathrin-mediated endocytosis, and (iii) ultrafast endocytosis [49][149]. Apart from those mechanisms, several other mechanisms, such as ultrafast bulk endocytosis and activity-dependent bulk endocytosis, are also responsible for the recycling of the vesicles [50][51][150,151]. Numerous proteins play vital roles in the mechanisms of endocytosis, including amphiphysin 1 (AMPH1), endophilin A1, clathrin, dynamin, and synaptojanin 1 (SYNJ1) [49][149]. Moreover, several regulatory proteins are also involved in the regulation of endocytosis. Collectively, these proteins function in an organized manner to propel various endocytic mechanisms, thus efficiently recycling the SVs, which is crucial for continuous supply of neurotransmitter-filled SVs, performance of sensory functions and maintenance of synaptic physiology [50][150]. These reports suggest that the process of endocytosis and associated proteins plays a significant role in neurotransmission at synapse and maintain the neural homeostasis.
Alterations in neuronal homeostasis and poor neuronal function lead to several pathological conditions collectively known as neurodegenerative diseases. In the current scenario, neurodegenerative diseases occur prominently in a large population of the world and pose a socio-economic burden [52][152]. Extensive studies suggest that the dysfunction of endocytosis signaling at synapse participates in the progression of various neurological disorders such as Alzheimer’s disease (AD), Parkinson’s disease, and Amyotrophic lateral sclerosis (ALS).
Alzheimer’s disease (AD) is the most prevalent type of neurodegenerative disease [53][153]. The major pathologies associated with AD include accumulation of amyloid-β (Aβ) plaques and development of neurofibrillary tangles (NFTs) due to hyper-phosphorylated tau protein [54][154]. Deregulation of endocytic processes such as CME and CIE accumulates the (Aβ) plaques and progresses AD [55][155]. The genome-wide association studies (GWAS) of AD patients revealed that the deregulated expression of genes PICALM, BIN-1, and sorLA, which are essentially involved in clathrin-mediated endocytosis [56][57][156,157]. In addition, the altered expression of Rab5, clathrin, dynamin 2, and PICALM has been found in transgenic Tg2576 mice AD experimental models [53][58][153,158]. Further, an elevated expression of caveolin-1 reported in the hippocampus and cortex regions of AD brains [59][159]. The endocytic signaling is considered as tightly regulated cellular process and vulnerable to hyperphosphorylated tau protein [60][160]. Apart from endocytic proteins and signaling, Endolysosomal or autophagic abnormalities play essential roles in the progression of AD pathologies [60][160]. The endocytic signaling is also involved in the internalization of extracellular Aβ that accumulates in endosomes and leads to neuronal toxicity [61][161]. Earlier evidence suggests that the amyloid precursor protein (APP) (a major component involved in the production of Aβ) could be internalized via CME and CIE pathways. Further, the expression level of endocytic proteins, APP, Tau proteins, and other molecules is varied with the age as well as AD progression. The expression of endocytic proteins such as AP180, caveolin-2, clathrin, dynamin-1, flotillin-2, and Rab-5 has been found significantly elevated, and that change accelerates endocytosis and progresses AD in aged brains [53][153]. Moreover, elevated tau protein induces microtubule assembly and sequesters free dynamins that impair the endocytosis and subsequently perturb the neurotransmission. The neural cells express N-methyl-D-aspartate (NMDA) receptors which are ionotropic glutamate receptors and regulate transmission of glutamate neurotransmitters. The surface expression of NMDA receptors is tightly regulated through clathrin-dependent endocytosis [62][63][162,163] and is shown to be endocytosed both in primary neuronal cultures and in vitro heterologous cells [62][162]. NMDA receptors play an important role in Aβ-induced neurotoxicity [62][162]. Moreover, Aβ regulates NMDA receptor response by promoting their endocytosis and are associated with synaptic transmission [64][164]. Therefore, the evidence suggests that the alteration in the endocytosis process and associated protein expression progresses AD.
Parkinson’s disease (PD) is the second most prevalent neurodegenerative disease associated with degeneration and subsequent loss of dopaminergic neurons [65][66][165,166]. The synaptic dysfunction is a key event prior to the loss of dopaminergic neurons and participates in the pathogenesis of PD. In a normal human brain, the neurotransmitters release as well as uptake and synaptic vesicles (SV) recycling occur in the synapse. The clathrin-mediated endocytosis participates in SV recycling [51][151]. Further, the process of synaptic vesicle endocytosis (SVE) regenerates a synaptic vesicle, which is a tightly regulated event and essential for neurotransmission. During PD, the synaptic dysfunction is associated with deregulated SVE signaling. Several genetic studies and mutation analysis suggest that the genes such as DNAJC6, SYNJ1, SH3GL2, SNCA, LRRK2, PRKN, and DJ-1 plays a vital role in the modulation of SVE and progression of PD [49][149]. The SVE dysfunction leads to erroneous dopamine packaging into the vesicles, as a consequence of elevated cytosolic dopamine and subsequent dopaminergic neurodegeneration [49][149]. Thus, deregulated expression of endocytic genes and SVE signaling perturb dopamine signaling and subsequent neurotransmission, which promotes the pathologies of PD. α-Synuclein (α-syn) is a 140-amino-acid soluble acidic protein highly expressed in pre-synaptic nerves has been implicated in the pathogenesis of PD [67][167]. α-syn can regulate clathrin-mediated endocytosis of membrane receptors [68][168] and is involved in the regulation of NMDA receptor endocytosis [69][169]. Further studies are needed to explore the mechanistic and therapeutic potential of targeting α-syn and NMDA receptors for the treatment of PD.
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease associated with the central nervous system (CNS). The major pathological condition of ALS is the degeneration of the motor neurons in CNS leading to muscles weakness [70][170]. Occurrence of ALS is sporadic and familial event, and multiple genes are involved in the progression of both types of ALS [71][171]. The genetic studies revealed that the mutation in the Chromosome 9 open reading frame 72 (C9ORF72) progresses ALS. Interestingly, the impaired endocytic signaling has been observed during C9ORF72 mutated conditions in C9ORF72 ALS/FTD patients as well as the SH-SY5Y cell line model, suggesting that the expression of C9ORF72 modulates endocytosis [72][73][172,173]. Further, the expression of C9ORF72 is associated with endocytosis of tropomyosin receptor kinase receptor B (TrkB) (essential for the development and functioning of nervous system) in neurons [70][170]. Earlier reports suggested that the valosin-containing protein (VCP/p97) regulates endolysosomal sorting of ubiquitylated caveolin-1 [74][174]. Another protein TDP-43 (nuclear RNA binding protein) inhibits endocytosis and localizes with endocytic proteins in tissue samples of ALS patients. In addition, dynamics of endocytosis modulate TDP-43 expression in a TDP-43 ALS fly model [75][175].
Collectively, these studies suggest that various endocytic pathways play an essential role in the progression of neurodegenerative diseases. Therefore, targeting the endocytic proteins and signaling mechanism associated with endocytic processes could be the effective approach to target neurological diseases for therapeutic intervention.
4. Inflammatory Bowel Diseases
Epithelial integrity and barrier function are critical to separate luminal contents, including nutrients and microbes from the underlying intestinal tissues [76][77][176,177]. Perturbations to the epithelial integrity are believed to contribute intestinal dysbiosis and allows heightened microbial penetration, resulting in chronic diseases such as inflammatory bowel disease (IBD), which is an umbrella term for Crohn’s disease (CD) and ulcerative colitis (UC) [78][79][178,179]. Endocytotic exodus of microbes in the barrier dysfunction CD, induces mucosal inflammation [80][81][180,181]. A case study of a 24-year-old woman with a positive family history of Crohn’s disease showed an increased intestinal permeability precedes the onset of Crohn’s disease [82][182]. Accumulating evidence shows that the intestinal barrier integrity is chiefly regulated by several multicomplex proteins, constituted of tight junctions (TJs) and adherens junctions (AJs) proteins [83][183]. Mammalian TJs have diverse roles, ranging from mediating selective diffusion of molecules across the epithelium to cis and trans interactions at sites of intercellular spaces [84][184]. The claudin protein family of TJs, including junctional adhesion molecule (JAM)-A, the tight junction-associated MARVEL proteins (TAMP), and coxsackievirus and adenovirus receptor (CAR), additionally consist of scaffolding molecules such as the zonula occludens (ZO) protein [85][185], chiefly regulates the organizational framework of intercellular barrier [86][186]. These claudin protein family are fundamental to establishing the paracellular passages of nutrients between the intestinal lumen and internal environment, as well as defense mechanisms against pathogens [87][88][187,188]. Dysregulated expression of sealing claudins and increased intestinal permeability contributes to a leaky epithelial barrier and may lead to intestinal infection and bowel symptoms of IBD patients [78][89][178,189]. The dextran sulfate sodium (DSS) colitis has been described as a most suitable in vivo experimental model of IBD to study intestinal barrier permeability and dissemination of microbes across the intestinal lumen [90][91][92][190,191,192]. Previously in the mouse DSS colitis model, a redistribution of occludin expression was observed compared to distinct appearance at the tight junctions of the apical membrane of colonic epithelium [93][193]. Apart from claudins, expression levels of other TJ proteins, such as JAM-A, occludin, and ZO-1, remain suppressed during intestinal inflammation [94][194].
Adherens junctions (AJs) are cell–cell adhesion complexes and usually annotated as “cadherins” [95][195]. Intestinal epithelial cells (IECs) largely express epithelial (E) cadherin, which is required to maintain colonic epithelial barrier permeability, and dysfunction can aggravate colitis [96][196]. Moreover, dysregulated expression of E-cadherin has been reported in tissue biopsies of IBD patients [97][197].
The proper assembly and functioning of junctional proteins are channelized through exocytic transportation of newly synthesized proteins to the cell surface and recycling of mature TJs and AJs via endocytosis [98][198]. The defects in TJs and AJs endocytosis leading to barrier disruption in IBD have been reported long ago [99][199]. The endocytosis of TJs and AJs happens similar to the internalization of luminal antigens into the enterocytes, and IBD patients have an increased ability to transcytosis of luminal antigens [89][189]. Importantly, recycling and exocytic trafficking of TJs and AJs proteins orchestrated through interaction of carrier vesicles and intracellular organelles [98][198]. Mechanistically, the membrane-targeted trafficking of carrier vesicles mediated through the protein family of Rab small GTPases and ultimate engrafting to lipid membrane is driven by the SNARE (soluble N-ethylmaleimide-sensitive factor associated receptor) proteins [100][200]. These results highlight an involvement of organelle-specific trafficking in the establishment of the intestinal epithelial barrier. The intracellular network of endoplasmic reticulum (ER)-Golgi trafficking controls synthesis and recycling of junctional proteins during intestinal inflammation [101][201]. Notably, the excessive fragmentation and vascularization are the characteristic dysfunction of the ER and the Golgi networks and observed in the intestinal mucosa of UC patients [102][202].
Altogether, an intact mucosal barrier restricts the infiltration of pathogenic microbes and regulates the absorption and passage of nutrients from the intestinal lumen into the underlying circulation. Endocytosis plays a critical role in the trafficking of intestinal junctional proteins TJs and AJs; however, dysfunction of the junctional proteins is a causative factor in the pathogenesis of IBD. Additional studies may further elucidate the mol
