Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Alaa A. A. Aljabali and Version 2 by Sirius Huang.
The interactions of nanoparticles (NPs) with cells of the immune system and their biomolecule pathways are an area of interest for researchers. It is possible to modify NPs so that they are not recognized by the immune system or so that they suppress or stimulate the immune system in a targeted manner.
- cytotoxicity
- oxidative stress
- reactive oxygen species
- nanomaterials
- immune system
1. Introduction
Our immune system has two main subsystems: the innate immune system and the adaptive immune system. The innate immune system is the first line of defense against foreign particles. It destroys infected cells or foreign material when it encounters them. This early, fast reaction is called the non-specific immunological response of the innate immune system. The innate immune system is fundamentally controlled by phagocytic cells (macrophages, dendritic (DCs), neutrophils, and mast cells (MCs)). The adaptive immune system works through specific cells, including T and B cells. Phagocytic cells, including antigen-presenting cells (APCs), destroy foreign antigens (bacteria, fungi, non-self-particles, etc.). T lymphocytes can also be divided into helper T cells (Th), which have subtypes called Th1, Th2, Th17, regulatory T cells (Tregs), and Th22 cells. Each subtype has owned its way to getting an immunological response from cells. For example, immune responses caused by Th1 cells are pro-inflammatory and involve cytokines such as interferon IFN-, interleukin (IL)-2, and tumor necrosis factor (TNF-α and TNF-β). Th2 cells are responsible for mediating a cellular anti-inflammatory antagonistic immune response [1].
Nanoparticles (NPs) can have positive or negative health impacts depending on their physical makeup, making them a “double-edged sword”. Physical-chemical properties, such as structural composition, surface charge, shape, crystallinity, surface area, zeta potential (surface charge), solubility, and surface functionalities, affect the NP toxicity. NPs should not be regarded as a homogeneous population with simple toxic properties because they independently mediate different biological reactions. Schrand et al. recently reviewed and outlined the toxicities of several metal-based nanoparticles, both in vitro and in vivo [2]. Although some nanomaterials are immunotoxins or immunomodulators, it would still be useful and vital for researchers to provide a concise overview of how NPs and the immune system interact.
2. NPs and the Immune System
The immune system’s primary function is to recognize and eliminate foreign agents. An inadequate immune system affects the quality of life. There are several ways in which immunity must be improved or toned to suppress pathogenic agents and help with autoimmune problems [3][5]. Innate immunity is the non-specific and primary line of defense in the body that relies on pattern recognition receptors (PRPs) to identify large molecular patterns present in pathogens and pathogen-associated molecular patterns (PAMPs) and is composed of various components, including serum protein, cells, and physical obstacles, which work together to defend against invaders [4][6]. The innate mechanisms of the immune system and its protection from foreign entities make it more responsive to the absorption of phagocytic cells. The inflammatory response is another critical component of the innate immune system [5][7].
Adaptive immunity can also be divided into humoral immunity and cell-based immunity. The former is responsible for antibody-mediated reactions, which analyze and neutralize their targets, whilst the latter oversees cytotoxic T cell-mediated direct killing of disease-affected endogenous cells (antigen-specific). Simultaneous stimulation of PAMP through the pattern recognition receptor promotes co-stimulatory factor expression, a favorable sign of antigen-specific T cells in concert with the representation of the corresponding peptide-MHC representation [6][8]. Although antigen fragments can only be found in all cells via class I MHC (MHC-I) molecules, antigens found only in qualified APC cells can be presented through class II molecules, which are required for humoral immunity and T-helper cell proliferation [6][7][8][8,9,10]. These product degradations are then transported to the endoplasmic reticulum and then presented on the cell’s surface to form complexes using MHC-Class I products. If the virus gene protein or other intracellular proteins are formed by cytoplasm, fragments of this protein are also expressed on the cell’s surface in complexes with MHC gene products of class I T cell receptors and are then identified as foreign molecules, and T-cells (CD8-positive) with receptors are triggered and kill target cells with the external antigen [9][11].
The antigen presentation process is central to adaptive immunity, providing the material required to control downstream immune effectors. APCs, including dendritic cells, are responsible for scavenging and eliminating foreign materials [10][12]. Excessive stimulation of immune reactions is undesirable and will have negative consequences. Therefore, the influence of the immune system must be considered when designing a nanomaterial for in vivo applications, as shown in Figure 1.
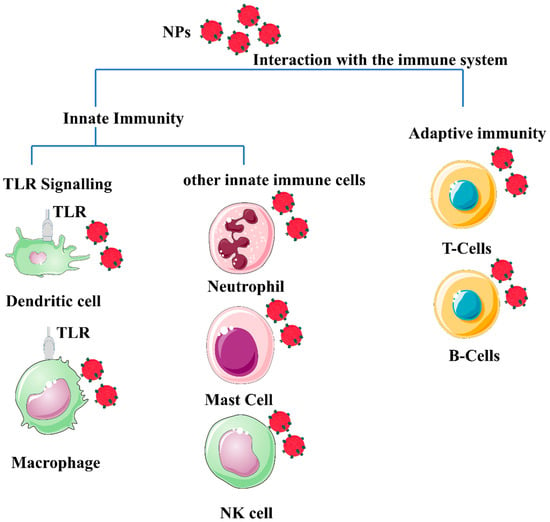
Figure 1. Schematic illustration of a generic form of nanomaterials (polymeric or metal-based) depicted in red spheres, showing their potential interaction with different immune system cells. The different properties of nanoparticles (NP) properties, such as morphology, size, surface charge, and composition, influence the interaction with immune cells. Most NPs induce an innate immune response. Mature innate immune cells such as lymphocytes, dendritic cells, monocytes, mast cells, neutrophils, macrophages, and natural killer cells, as well as pattern recognition receptors such as toll-like receptors (TLRs). In addition, nanomaterials’ interaction with the adaptive immune system activates Th1/Th2 responses and stimulates the production of cytokines. The effect of nanomaterials on B cells appears to enhance immunity during vaccination, and B cells are normally targeted by specifically functionalized nanomaterials to target B cells with lymphoma to kill lymphoma and generate a chemotherapeutic effect from the use of the NPs. The image was generated by BioRender.
Once a nanomaterial is designed for use in vivo, three immune-related effects should be considered. The first is degradation or rejection through the immune system, which may result in a protective immune response. The second point is immunotoxicity, which could damage and trigger inflammatory and pathological changes in the immune system. Third, immune compatibilities do not affect the immune response [11][12][3,13].
3. Nonspecific Immunomodulation
NPs were used as a cytokine delivery tool, helped modulate physical localization, and served as a repository for sustainable release. The justification was to help effectively manage the nontarget cytotoxicity associated with these treatments.3.1. Nanomaterials and Immune System Modulations
The sheer quantity of physicochemical properties of NPs, including shape, size, morphology, and elemental constituents, makes the investigation of their cytotoxicity consequences complex and challenging. Oxidative stress, inflammation, genetic damage, cell division, and cell death suppression are paradigms for NP-mediated toxicity. Considerable existing research has revealed that NP cytotoxicity is commonly associated with ROS production (which may be either protective or harmful during biochemical processes), and subsequently, oxidative stress is frequently reported with NPs toxicity [13][14]. ROS includes superoxide radicals (O2•), hydrogen peroxide (H2O2), hydroxyl radicals (•OH), and singlet oxygen (1O2). Biological systems produce them as metabolic by-products. Adequate oxidative stress is the foundation for many other activities, including induction of protein phosphorylation, multiple transcription factor activation, cell apoptosis, host defense, and differentiation [14][15]. Intrinsically or externally, ROS can be synthesized inside the cell. Molecular oxygen is generated by O2•, and primary ROS is mediated by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase through a single electron decrease. Using a metal to speed up the Fenton reaction, a further reduction in oxygen could change H2O2 or •OH into something else [15][16][16,17]. Inflammatory phagocytes, such as neutrophils and macrophages, generate oxidative outbursts as a defensive mechanism against environmental contaminants, tumor cells, and microorganisms. Intracellular calcium levels activate transcription factors and modulate cytokine synthesis by mechanisms of free radicals and therefore contribute to the influence of NP on oxidative stress [17][18]. Glutathione-S-transferase (GST) is a phase II enzyme family that catalyzes electrophilic detoxification and protects cells from mutagens, carcinogens, and glutathione, all of which act as free radical scavengers. GST in the cytosol eliminates H2O2. GST interacts with H2O2 in a glutathione peroxidase catalyzed chemical reaction that results in glutathione disulfide (GSSG). Glutathione reductase (GR) catalyzes GSSG glutathione regeneration using the NADPH hexose monophosphate shunt (HMPS) produced by NADPH [18][19]. NP-mediated ROS responses orchestrate a cascade of adverse pathological processes such as inflammation, genotoxicity, fibrosis, and carcinogenesis. Nanomaterials with different chemical compositions, such as fullerenes, CNTs, and metal oxides, have been reported to promote ROS. The major variables in NP-induced ROS are (i) active redox cycling on the surface of NP due to transition metal-based NP, (ii) surface functional groups, and (iii) NPs–cell interactions [19][20]. Furthermore, CNT-induced oxidative stress, for example, promotes cellular signaling pathways, increasing the production of pro-inflammatory cytokines. Some NPs have been shown to activate inflammatory cells, such as macrophages and neutrophils, increasing ROS production. Several NPs, such as titanium dioxide (TiO2), zinc oxide (ZnO), cerium oxide (CeO2) and silver NP, have been documented to deposit on the cellular surface or even inside intracellular organelles and trigger oxidative stress signaling cascades, resulting in oxidative damage to the cell [20][21]. Because of their surface characteristics, NPs, such as Si and Zn with identical particle sizes and shapes, exhibit varied cytotoxicity responses. Since ZnO is more chemically active than SiO2, it generates more free radicals, leading to increased oxidative stress [21][22]. The process of ROS formation differs for each NP, and the precise underlying cellular mechanism for ROS generation is still unknown and must be explored [22][23].3.2. Immunological Effects of Nanomaterials (Immunotoxicology)
When used in vivo, NPs and the interface between the NP and the biological material play a key role in how the nanomaterial is transported, eliminated, and deposited, especially when it comes to the systemic administration of medicines. A protein crown effect, also known as a protein corona particle, is formed when NPs encounter biofluids for the first time. The way in which NPs interact with plasma proteins and other biomolecules changes their bioactivity, which in turn causes several changes in the way the body works. When the immune system encounters a foreign body, such as NPs, the first cells to respond are phagocytic cells. Several conditions, including inflammation, are caused by overactive immune systems, whereas immunosuppression leaves the host susceptible to infections by the inhalation of CNTs was used to decrease B cell activity via NP involvement in immunosuppression [23][24]. The alveolar macrophage produced is important for the immunosuppressive process observed. When the immune system encounters a foreign body, such as NPSs, the first cells to respond are phagocytic cells. There have been several reports of negative interactions between the immune system and nanoparticles, with immune stimulator immunosuppression potentially leading to inflammatory or autoimmune diseases and increasing the receptor likelihood that the body will get infected. Immunosuppressive NPs were used to reduce B cell activity by allowing people to breathe in CNTs. However, when a nanomaterial is administered subcutaneously or intradermally, stimulation of the complement system by NPs can improve the effectiveness of the treatment. Modifying the physicochemical features of NPs allows for control over the pathways by which they activate the complement system. After being exposed to nanoparticles, important biological processes happen, and mast cells play a role in inflammation and the toxicity of some NPs [24][25][25,26].3.3. Mechanism of Nanomaterials Toxicity
Direct or indirect modulation of oxidative enzymatic reactions by NPs causes oxidative stress because of free radicals, or ROS, such as superoxide anions and hydroxyl radicals. Multiple factors contribute to oxidative stress in the body. Cellular responses to nanoparticles are accompanied by the production of ROS, which can be stimulated by the oxidant characteristics of the particles themselves. Free radical intermediates are present on the reactive surfaces of particles and are quite stable; however, there are effects of NP functionalities on the formation of redox-active groups. A summary of the influence (immunogenicity, antigenicity, clearance, immunostimulation, and immunosuppression) of nanomaterials on the immune system is shown in Figure 2.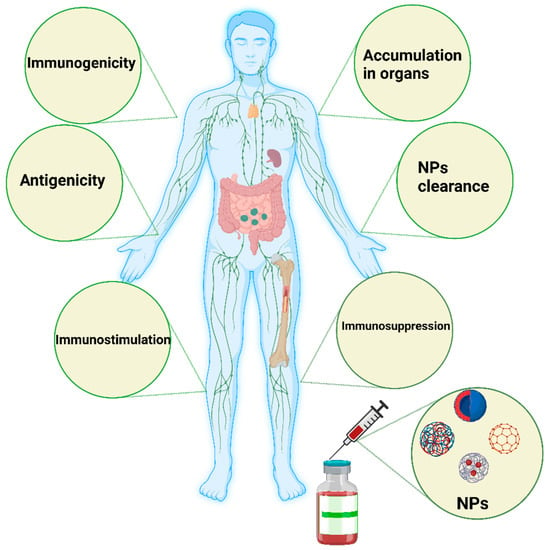
Figure 2. Schematic illustration of the major influence of nanomaterials on the immune system. Nanomaterials can induce immunogenicity, which is the ability of NPs to induce humoral or cell-mediated immune responses, whereas the antigenicity effect of NPs deals with the ability of such materials to react with antibodies or T cells. NPs could be engineered to avoid interactions with the immune system or specifically interact with the immune system to simulate or suppress their effects. In some cases, the immune system stimulation generated by such NPs could manifest as a hypersensitivity reaction, anaphylactic shock, or inflammation. In immunosuppression, NPs can have a stimulating effect on immune system components, such as APCs, B cells, and T cells, directly or indirectly. The degradation and clearance of NPs are the main factors contributing to the use of such materials in clinical settings. Tiny NPs below 10 nm are rapidly excreted by the kidney and liver. Particles larger than 100 nm can be eliminated by the mononuclear phagocytic system, such as the lymph nodes and spleen. The image was generated by BioRender.
3.4. Nanomaterials Affect Cell Functions
The practical problem of immunosuppression and immunostimulants mediated by NPs is highly innovative, as the interaction of NPs with the body’s immune system and their impact have not been fully understood; nevertheless, there is a considerable amount of evidence that illustrates the enormous potential of these immunomodulation strategies for the treatment of immunological disorders. The use of NPs and the modulation of immunostimulants and immunosuppression are summarized in Table 1.Table 1.
Immune system modulation by NPs.
| NPs Effect | Immunosuppression | Immunostimulant | References |
|---|---|---|---|
| Desirable | Reducing allergic response | Used as cancer immunotherapy | [26][27[27],28] |
| Organ and tissue transplant rejection | Enhanced vaccine efficacy | [28][29] | |
| Very useful for autoimmune disorders and inflammation treatment | Antitumorigenic | [29][30] | |
| Undesirable | Severe suppression of the immune system to a level affecting the recognition | Development of inflammation | [30][31] |
| Myelosuppression (bone marrow and thymus gland) | Development of hypersensitivity | [31][32] | |
| Development of anaphylaxis | [26][27] |
3.5. Immunosuppression
Immunosuppression occurs when a medication or other substance inhibits the activation or effectiveness. Immunosuppression is commonly thought to be an immunotoxic impact linked to various human diseases. The size, morphology, and composition facilitate blood, cellular, and protein adsorption, allowing for more efficient interactions with immune cells and the subsequent immunological response. AuNPs are an excellent example of these effects. Larger AuNPs and rod-shaped geometries are routinely internalized via sophisticated uptake mechanisms. Spherical NPs between 5 and 30 nm in diameter are susceptible to passive association with cells. The surface coating does have an impact on their absorption by cells. For example, citrate or lipid capping of the AuNPs may increase the particles’ stability and passive intake [32][33][33,34]. On the other hand, since silver and gold NPs interact with adaptive and innate immune systems, few studies have elucidated the mechanism behind the potential of noble metal NPs to induce an immunosuppressive response [34][35]. Citrate-coated AuNPs have been shown to have no measurable cellular or organ toxicity in mice. However, researchers reported anti-inflammatory potential that reduced cellular responses triggered by interleukin 1 beta (IL-1β). IL-1 β is an inflammatory cytokine that mediates adaptive immune responses, as well as common inflammatory conditions such as rheumatoid arthritis [35][36][36,37]. The potential of monodispersed citrate-coated AuNPs varying in size from 5 to 35 nm to regulate pro-inflammatory functionality induced by IL-1β generation was examined. The IL-1β pathway disrupted the smallest nanoparticles, measuring 5 nm. Larger nanoparticles (>10 nm) had less of an impact, while 35 nm particles did not affect the IL-1 pathway [37][38]. Furthermore, poly(acrylic acid)-coated AuNPs have been shown to have opposite effects in the same THP-1 cell lines by promoting inflammation [38][39]. Immunosuppression produced by AgNPs has received less attention than AuNPs. Silver nanoparticles stimulated the production of TNF-, IL-6, IL-8, IL-1, and IL-11 production [39][40]. Tian et al. demonstrated that topical treatment of AgNPs modulated cytokines at a wound site. IL-6 mRNA expression was considerably reduced during the healing phase, whereas TGF-1 expression increased. It has also been demonstrated that local and systemic applications can reduce inflammation [40][41]. In addition, iron oxide NPs have been shown to induce immunosuppression and anti-inflammatory activities. Ovalbumin-sensitized mice received ovalbumin, a T cell-dependent antigen, after an administration of iron oxide nanoparticles in investigations by Liao et al. and Shen et al. Antigen-specific antibody expression, as well as antigen-specific cytokine secretion in the spleen, was considerably decreased. Iron oxide nanoparticles inhibited the activity of T helper cells and macrophages while decreasing interferon, IL-6, and TNF-expression [41][42][42,43]. TNF- and IL-6 are both cytokines that contribute to inflammatory responses. Iron oxide NPs also changed the proportion of helper T cells, suppressing the allergic response. In an alternative animal model, administration of iron oxide particles preceding immunological activation with an endotoxin lowered IL-1 expression in microglia cells. Iron oxide NPs hindered cytokine processing pathways, culminating in the attenuation of IL-1. Human dendritic cells treated with poly(vinyl alcohol)-coated iron oxide NPs exhibited impaired antigen processing and T cell activation in a separate study. Injections of iron oxide nanoparticles were given again, and the inflammation was reduced [43][44]. According to Jan et al. [44][45], iron oxide NPs accumulated in lysosomes, improving lysosomal accessibility, and reducing cathepsin B activity. Furthermore, cerium can consistently switch redox potential between Ce4+ and Ce3+, allowing cerium oxide NPs to absorb reactive oxygen species efficiently. As cerium oxide switches between oxidation states, it creates oxygen vacancies in the crystal lattice. Since the vacancies are clustered at the NPs’ surfaces, cerium oxide NPs have a high capability to interact with free radicals immediately. This antioxidant potential has been demonstrated in several studies [44][45]. Cerium oxide NPs antioxidant properties allow them to suppress inflammation caused by nitric oxide synthase production [45][46]. Superoxide dismutase-2, an oxidative stress mediator, was also upregulated by cerium oxide NPs. The antioxidant properties of cerium oxide NPs, similar to those of many other NPs, are influenced by their size and morphology. As a result, smaller cerium oxide NPs outperformed their larger counterparts in antioxidant capacity. Figure 3 summarizes the various effects of the NPs on different immune cells, depending on whether immunosuppressive or inflammatory cytokines are secreted.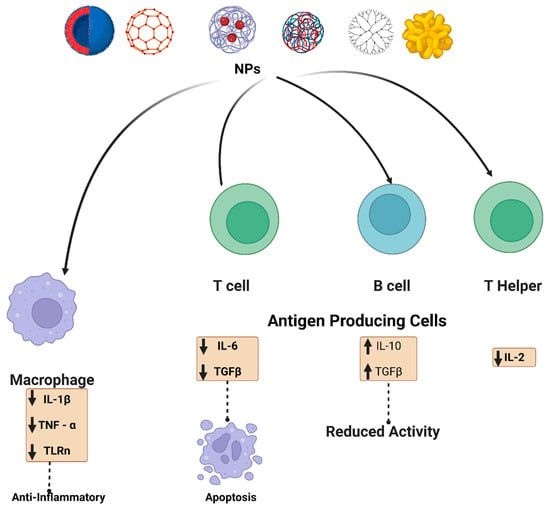
Figure 3. Schematic illustration of the NPs interaction and effect on the immune system. NPs (metalic, polymeric, dendrimer, carbon-based and lipid-based) interact with immune cells such as antigen-presenting cells, B cells, T cells, and macrophages. For example, carbon-based nanomaterials are involved in up-regulation of transforming growth factor (TGF-β), interleukin-10 (IL-10), and it also decreased B cell activity in addition to apoptosis. Metal oxide NPs have also been reported to affect adaptive immune cells, such as cerium oxide NPs, which are responsible for scavenging reactive oxygen species (ROS). Polymeric NPs and dendrimers exhibit an assortment of immunosuppressive effects. Some possible mechanisms by which various nanoscale compounds might dampen the immune system are illustrated.
3.6. Immunostimulant
The propensity of NPs to induce innate or adaptive immune responses is being used to assess their immunostimulatory potential as summarize in Table 2. The stimulation of the complement cascade can be detrimental if particles penetrate the systemic circulation accidentally or on purpose, resulting in hypersensitivity responses and anaphylaxis [65][66]. The size has been proposed to be an important factor in determining whether antigens loaded into nanoparticles produce type I (interferon-) or type II (IL-4) cytokines, thus contributing to the type of stimulatory immune response [66][67]. Induction of allergic responses is one aspect of NP-mediated immunostimulation. A few investigations have connected NP exposure to allergy responses in test animals and humans. In mice, for example, SWCNT and MWCNTs equally increased the allergenicity of egg albumin, whether administered by intranasal or subcutaneous injection routes. The CNT-mediated activation of the acute inflammatory response is hypothesized to be the mechanism underlying this enhanced allergenicity [67][68]. In rare circumstances, nanoformulation of an authorized treatment would prevent allergic responses linked to previously approved preparations, and abraxane is an illustration of this reformulation. In this instance, reconstituted paclitaxel-bound albumin nanoparticles provoked no allergic response, but the first-generation formulations of paclitaxel in the non-ionic surfactant Cremophor EL triggered significant hypersensitivity, commonly involving premedication with only steroids and a histamine blocker [68][69]. Almost all the immunostimulatory responses elicited by nanoparticles are mediated by the release of pro-inflammatory cytokines. Numerous studies have provided evidence through various forms of nanomaterials that can induce cytokine production, i.e., AuNPs, lipid-based NPs, dendrimers, etc. [69][70][71][70,71,72]. The diameter of NPs has been perceived as a determinant factor in influencing an NP’s ability to generate cytokine activation. For example, CNT length has been reported to correlate with in vivo subcutaneous injection inflammation caused by CNT administration [72][73]. On the contrary, some studies have demonstrated that surfactants or microbial endotoxins included in the formulations, rather than NPs, trigger cytokine production. As a result, when analyzing the inflammatory properties of NPs, it is vital to evaluate the levels of chemical (byproduct generation) and biological (endotoxin) components.Table 2.
NP-based immunostimulatory effect and study results from the studies outcome.
| Type of NPs | Study Outcomes | References |
|---|---|---|
| Liposomes decorated with synthetic long peptides antigen | The immunological response was mediated by antigen-specific CD8+ T cells that were induced | [73][74] |
| Liposomes | Induction of antigen-specific response | [74][75] |
| Liposomes loaded with cytosine-phosphate-guanine and 3,5- didodecyloxybenzamidine |
DCs were stimulated to release cytokines, co-stimulatory molecules were expressed, and an antigen-specific immune response was enhanced | [75][76] |
| AuNPs | Macrophage activation | [76][77] |
| AuNPs loaded with BSA antigen | Anti-BSA antibodies were detected in greater concentrations in the blood serum of mice inoculated with BSA–AuNPs and cytosine-phosphate-guanine –AuNPs conjugates | [77][78] |
| MWCNTs | Inducing strong CD4+ T and CD8+ T- cells mediated immune response | [78][79] |
| MWCNTs loaded with anti-CD40 Ig | In subcutaneous or lung pseudo-metastatic tumor models, it increased entrapped ovalbumin-specific T cell responses and suppressed the development of entrapped ovalbumin–expressing B16F10 melanoma cells. | [79][80] |
| MWCNTs loaded with ovalbumin | Elicited a strong anti-tumor immune response | [80][81] |
| MWCNTs loaded with cytosine-phosphate-guanine | Elicited a strong cellular and humoral immune response | [81][82] |
| Iron oxide (Fe3O4) NPs | In vitro, it induced a significant adaptive immune response by stimulating DCs and macrophages, and it reduced tumor development and prevented tumor formation in vivo | [82][83] |
| Iron oxide (Fe3O4) NPs | Enhanced T cell activation and elevated stimulation of anti-tumor activity | [83][84] |
| Micelles loaded with cytosine-phosphate-guanine and Trp2 | In tumor-bearing mice, it produced antigen-specific cytotoxic CD8+ T cell-mediated immunity, as well as a robust anti-cancer immune response | [84][85] |
| Micelles loaded with cytosine-phosphate-guanine and Trp2 | Trp2/PHM10/ cytosine-phosphate-guanine nanoformulation dramatically increased CD8+ T cellular activities while improving anti-tumor effectiveness. | [85][86] |
| Micelles loaded with ovalbumin and CL264 agonist | A robust antigen-specific cellular and humoral immune response was elicited | [86][87] |
| Dendrimers loaded with ovalbumin and cytosine-phosphate-guanine | Elicited a much greater T-cell-mediated immunological response | [87][88[88],89] |
| Dendrimers loaded with cytosine-phosphate-guanine | Cytosine-phosphate-guanine delivered effectively into DCs triggered an adaptive cellular immune response | [89][90] |
| Protein NP loaded with melanoma-associated gp100 epitope and cytosine-phosphate-guanine | Antigen-specific antitumor immune response substantially increased | [90][91] |
| NP protein loaded with peptide epitope cytosine-phosphate-guanine | Elevated CD8+ T cell activation and antigen cross-presentation | [91][92] |
