Native Mexican plants are a wide source of bioactive compounds such as pentacyclic triterpenes. Pentacyclic triterpenes biosynthesized through the mevalonate (MVA) and the 2-C-methyl-D-erythritol-phosphate (MEP) metabolic pathways are highlighted by their diverse biological activity. Compounds belonging to the oleanane, ursane, and lupane groups have been identified in about 33 Mexican plants, located mainly in the southwest of Mexico. 45 compounds pentacyclic triterpenes were reported showing antiinflammatory, cytotoxic, anxiolytic, hypoglycemic, and growth-stimulating or allelopathic activities. Extraction is essentially performed by maceration and Soxhlet with organic solvents and consecutive chromatography of silica gel have been used for their whole or partial purification. Nanoparticles and nanoemulsions are the vehicles used in Mexican formulations for drug delivery of the pentacyclic triterpenes until now. Sustainable extraction, formulation, regulation, isolation, characterization, and bioassay facilities are areas of opportunity in pentacyclic triterpenes research in Mexico while the presence of plant and human resources and traditional knowledge are strengths.
Native Mexican plants are a wide source of bioactive compounds such as pentacyclic triterpenes. Pentacyclic triterpenes biosynthesized through the mevalonate (MVA) and the 2-C-methyl-D-erythritol-phosphate (MEP) metabolic pathways are highlighted by their diverse biological activity. Compounds belonging to the oleanane, ursane, and lupane groups have been identified in about 33 Mexican plants, located mainly in the southwest of Mexico. 45 compounds pentacyclic triterpenes were reported showing antiinflammatory, cytotoxic, anxiolytic, hypoglycemic, and growth-stimulating or allelopathic activities. Extraction is essentially performed by maceration and Soxhlet with organic solvents and consecutive chromatography of silica gel have been used for their whole or partial purification. Nanoparticles and nanoemulsions are the vehicles used in Mexican formulations for drug delivery of the pentacyclic triterpenes until now. Sustainable extraction, formulation, regulation, isolation, characterization, and bioassay facilities are areas of opportunity in pentacyclic triterpenes research in Mexico while the presence of plant and human resources and traditional knowledge are strengths. The present review discusses the generalities of the pentacyclic triterpene (definition, biogenic classification, and biosynthesis), a summary of the last two decades of research on the compounds identified and their evaluated bioactivity, the generalities about the extraction and purification methods used, drug delivery aspects, and a critical analysis of the advantages and limitations of research carried out in this way.
- pentacyclic triterpenes
- Mexican plants
- biological activity
- extraction
- purification
- drug delivery
1. Introduction
1.1. General Overview of Pentacyclic Triterpenes
1.1.1. Structure, Classification, Distribution, and Biological Importance
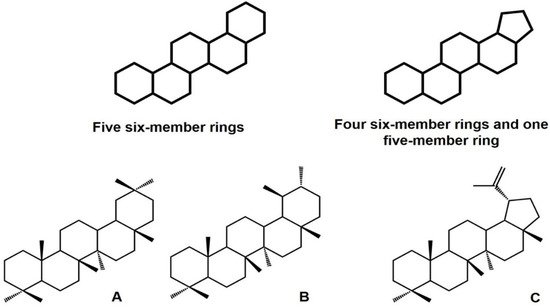
1.1.2. Biosynthesis
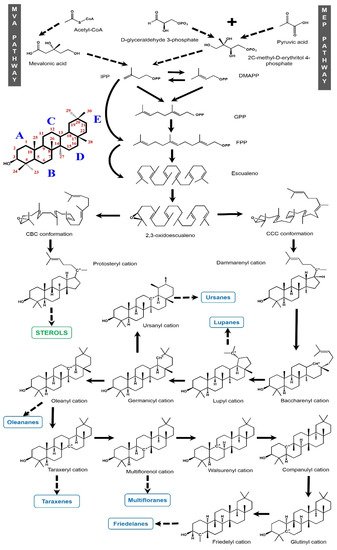
2. Pentacyclic Triterpenes from Mexican Plants
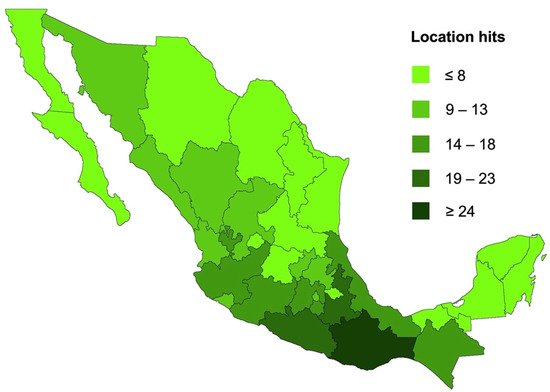
Biological Activity Survey of PCTs Found in Mexican Plants
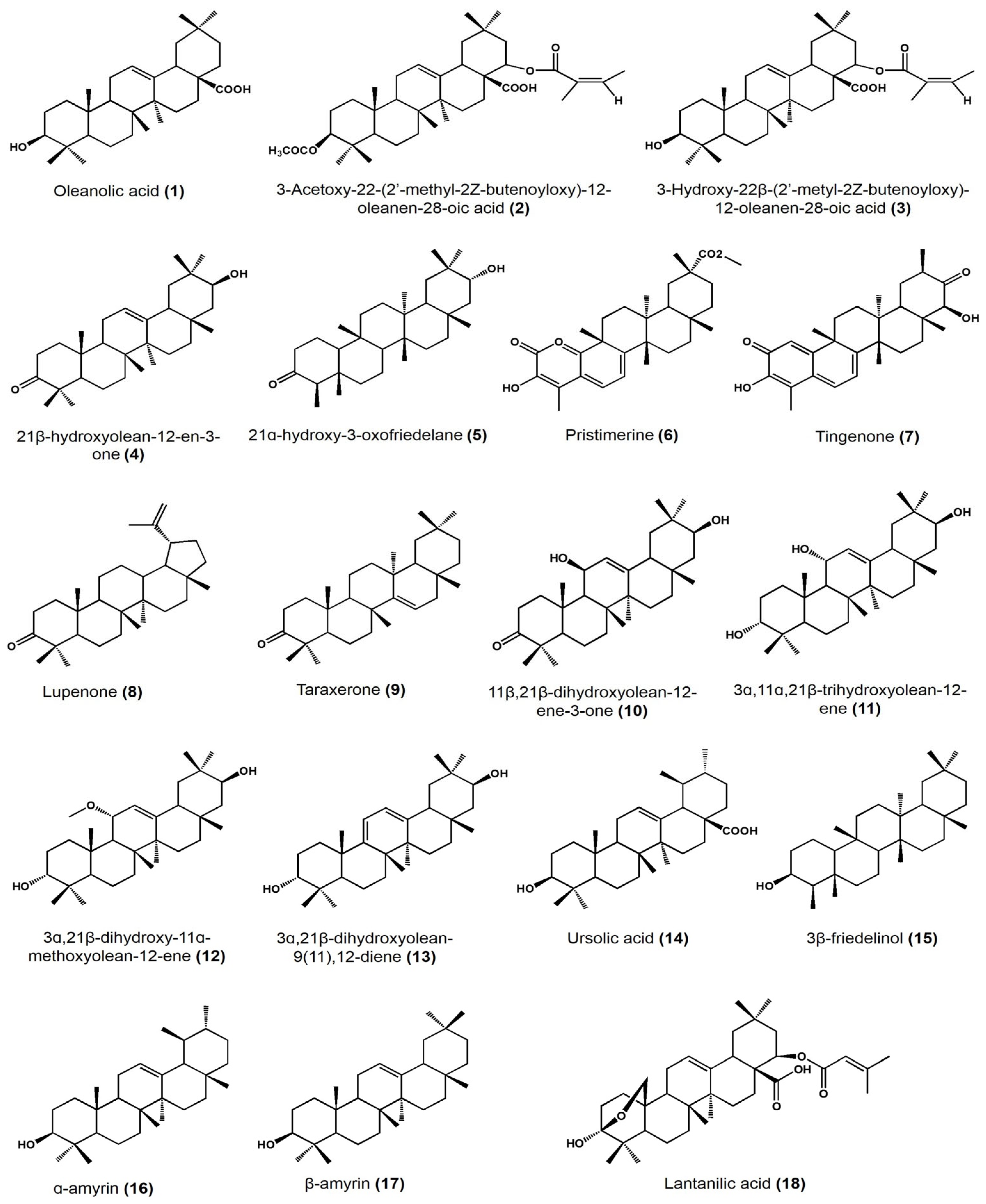
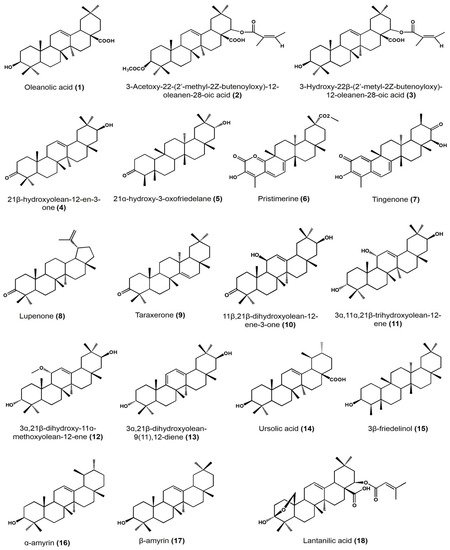
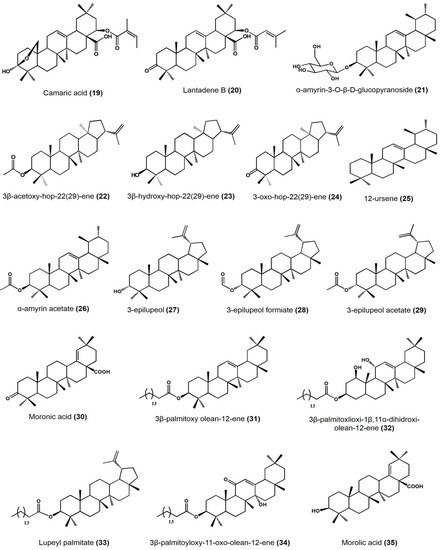
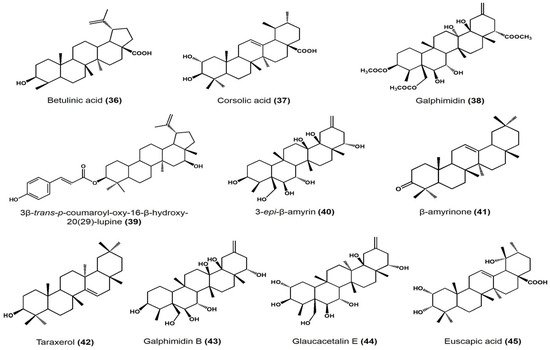
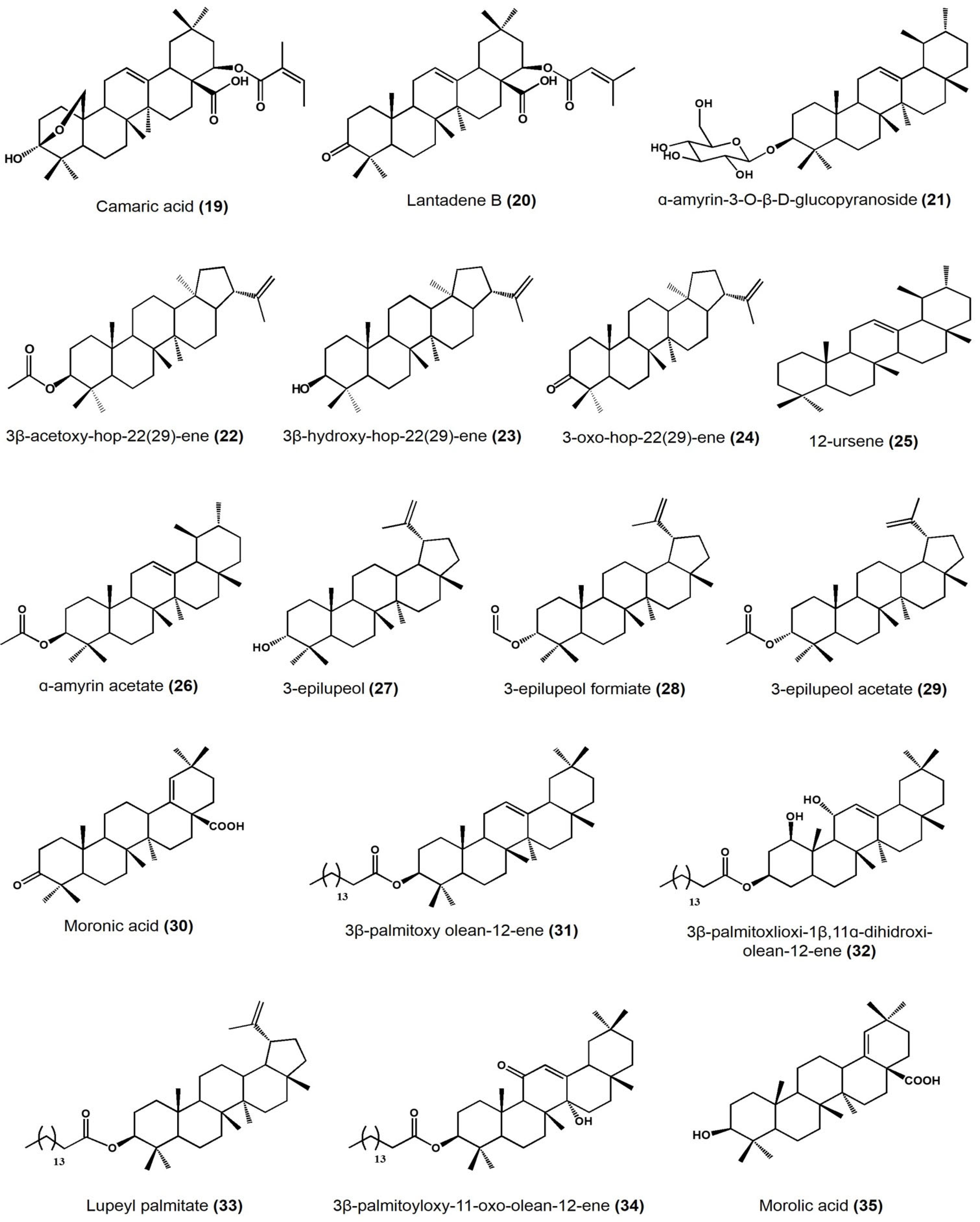
| Plant Species | Identified Compounds | Subgroup | Activities | References |
|---|---|---|---|---|
| Lantana hispida | Oleanolic acid | Oleanane | Antimicrobial activity (MIC) on Mycobacterium tuberculosis: • H37Rv: 25 μg/mL • STR-R, RIF-R, INH-R, and EMB-R: 50 μg/mL |
[29] |
| 3-acetoxy-22-(2′-methyl-2Z-butenyloxy)-12-oleanene | Oleanane | Antimicrobial activity (MIC) on M. tuberculosis: • H37Rv, STR-R, and INH-R: 25 μg/mL • RIF-R and EMB-R: 50 μg/mL |
||
| 3-hydroxy-22-(2′-methyl-2Z-butenoyloxy)-12-oleanen-28-oic acid (reduced lantadene A) | Oleanane | Antimicrobial activity (MIC) on M. tuberculosis: • H37Rv, STR-R, RIF-R, INH-R, and EMB-R: 50 μg/mL |
||
| Hippocratea excelsa | 21β-hydroxyolean-12-en-3-one | Oleanane | Antiprotozoal activity (IC50) on Giardia intestinalis: 27.4 μM | [30] |
| 21α-hydroxy-3-oxofriedelane | Friedelane | Antiprotozoal activity (IC50) on G. intestinalis: 19.8 μM | ||
| Pristimerine | Friedelane | Antiprotozoal activity (IC50) on G. intestinalis: 0.11 μM | ||
| Tingenone | Friedelane | Antiprotozoal activity (IC50) on G. intestinalis: 0.74 μM | ||
| Acacia cochliacantha | Lupenone | Lupane | Antimicrobial activity (MIC) on: • Staphylococcus aureus: 2.8 mg/mL • Bacillus subtilis: 1.4 mg/mL • Enterococcus faecium: 5.6 mg/mL • Lactobacillus plantarum: 11.3 mg/mL • Escherichia coli: 2.8 mg/mL • Salmonella typhirium: 22.5 mg/mL • Klebsiella pneumoniae: 22.5 mg/mL |
[31] |
| Taraxenone | Taraxerane | Antimicrobial activity (MIC) on: • S. aureus: 0.4 mg/mL • B. subtilis: 1.4 mg/mL • L. plantarum: 1.4 mg/mL • E. coli: 0.2 mg/mL • S. typhirium: 2.8 mg/mL • K. pneumoniae: 1.4 mg/mL • Pseudomonas aeruginosa: 2.8 mg/mL |
||
| Hippocratea excelsa | 11β,21β-dihydroxy-olean-12-ene-3-one | Oleanane | Antiprotozoal activity on Giardia intestinalis: IC50: 184.6 μM | [32] |
| 3α,11α,21β-trihydroxy-olean-12-ene | Oleanane | Antiprotozoal activity on G. intestinalis: IC50: 96.8 μM | ||
| 3α,21β-dihydroxy-11α-methoxy-olean-12-ene | Oleanane | Antiprotozoal activity on G. intestinalis: IC50: 690.7 μM | ||
| 3α,21β-dihydroxy-olean-9(11),12-diene | Oleanane | Antiprotozoal activity on G. intestinalis: IC50:78 μM | ||
| Chamaedora tepejiliote | Ursolic acid | Ursane | Antimicrobial activity (MIC) against M. tuberculosis: • H37Rv, INH-R, RIF-R, EMB-R, MMDO, and MTY147: 25 μg/mL • STR-R: 12.5 μg/mL In vitro intracellular load of M. tubrculosis MDR and H37Rv in macrophages at: • 6.25 μm/mL (14) + 12.5 μm/mL (1): (after 48 h) approximately 101 CFU/mL (both strains) • 0.625 μm/mL (14) + 1.25 μm/mL (1): (after 48 h) approximately 101 CFU/mL (H37Rv) Bacilli load per lung in mice model after 60 days of administration of 1:3 mixture of (1):(14) at 5 mg/kg • 0.1 × 106 bacilli/lung approximately • Control: approximately 0.9 × 106 bacilli/lung |
[33] |
| Lantana hispida | Oleanolic acid | Oleanane | Antimicrobial activity (MIC) against M. tuberculosis: • H37Rv, STR-R, MMDO, and MTY147: 50 μg/mL • INH-R, RIF-R and EMB-R: 25 μg/mL In vitro intracellular load of M. tubrculosis MDR and H37Rv in macrophages at: • 6.25 μm/mL: (after 24 and 48 h) approximately 105 CFU/mL. • 0.625 μm/mL: (after 48 h) approximately 105 CFU/mL • Control: approximately 106 CFU/mL Bacilli load per lung in mice model after 60 days of administration of 1:3 mixture of (1):(14) at 5 mg/kg • 0.1 × 106 bacilli/lung approximately• Control: approximately 0.9 × 106 bacilli/lung |
|
| Heterotheca inuloides | 3β-friedelinol | Friedelane | Antimicrobial activity (MIC) on Helicobacter pylori: >31.25 μg/mL | [34] |
| α-amyrin and β-amyrin | Ursane | |||
| Lantana camara | Lantanilic acid | Lantadene | Lehismaniacidal activity on L. mexicana: 9.50 ± 0.28 μM | [35] |
| Camaric acid | Lantadene | Lehismaniacidal activity on L. mexicana: 2.52 ± 0.08 μM | ||
| Lantadene B | Lantadene | Lehismaniacidal activity on L. mexicana: 23.45 ± 2.15 μM | ||
| Cisus incisa | α-amyrin-3-O-β-D-gluco pyranoside | Ursane | Antimicrobial activity (MIC) against carbapenems-resistant P. aeruginosa: 100 μg/mL | [36] |
| Cnidosculus spinosus | 3 β-acetoxy-hop-22(29)-ene | Hopane | Inhibitory activity on mouse edema at 0.31 μmol/ear: 57.27 ± 16.99% Inhibition of yeast α-glucosidase at 100 μM: 98.79% |
[37] |
| 3β-hydroxy-hop-22(29)-ene [Hopenol B] | Hopane | Inhibitory activity on mouse edema at 0.31 μmol/ear: 27.05 ± 7.38% Inhibition of yeast α-glucosidase at 100 μM: 23.47% Antiparasitic activity on Trypanosoma cruzi at 50 μM: <20% |
||
| 3-oxo-hop-22(29)-ene | Hopane | Inhibitory activity on mouse edema at 0.31 μmol/ear: 17.5 ± 4.11% Inhibition of yeast α-glucosidase at 100 μM:7.39% Antiparasitic activity on Trypanosoma cruzi at 50 μM: <20% |
||
| Agarista mexicana | 12-ursene | Ursane | Hypoglycemic activity on mice models after at 50 mg/kg: • 25.9 ± 6.1% glucose reduction |
[38] |
| Bursera copallifera | lupenone | Lupane | Anti-inflammatory activity in mouse edema inhibition at 1 mg/ear: 57.25 ± 1.36%, ID50: 1.052 μmol/ear Reduces NO production in LPS-stimulated macrophages: IC50: 20.08 ± 1.07 μM |
[39] |
| Bursera copallifera | α-amyrin | Ursane | Anti-inflammatory activity in mouse edema inhibition at 1 mg/ear: 25 ± 1.81%, ID50: >2.34 μmol/ear Reduces NO production in LPS-stimulated macrophages: IC50: 8.98 ± 1.73 μM |
[39] |
| α-amyrin acetate | Ursane | Anti-inflammatory activity in mouse edema inhibition at 1 mg/ear: 69.45 ± 1.8%, ID50: 1.17 μmol/ear Reduces NO production in LPS-stimulated macrophages: IC50: 22.47 ± 1.19 μM |
||
| 3-epilupeol | Lupane | Anti-inflammatory activity in mouse edema inhibition at 1 mg/ear: 66.39 ± 4.38%, ID50: 0.83 μmol/ear Reduces NO production in LPS-stimulated macrophages 15.50 ± 1.14 μM |
||
| 3-epilupeol formiate | Lupane | Anti-inflammatory activity in mouse edema inhibition at 1 mg/ear: 62.16 ± 1.8%, ID50: 0.96 μmol/ear Reduces NO production in LPS-stimulated macrophages 43.31 ± 2.60 μM |
||
| 3-epilupeol acetate | Lupane | Anti-inflammatory activity in mouse edema inhibition at 1 mg/ear: 49.35 ± 3.6%, ID50: >2.13 μmol/ear Reduces NO production in LPS-stimulated macrophages: IC50: 31.13 ± 1.25 μM |
||
| Bursera cuneata | α-amyrin | Ursane | Anti-inflammatory activity in mouse edema inhibition at 0.1 mg/ear: 44.9 ± 1.2% | [40] |
| Moronic acid | Oleanane | Anti-inflammatory activity in mouse edema inhibition at 0.1 mg/ear: 68.1 ± 1.3% Histamine inhibition in a mouse model at 0.1 mg/ear: 73.3 ± 1.1% Macrophages viability reduction at 60 μg/mL: 43% |
||
| Ursolic acid | Ursane | Anti-inflammatory activity in mouse edema inhibition at 0.1 mg/ear: 55.6 ± 2.1% |
||
| Sapium lateriflorum | 3β-palmitoyloxy olean-12-ene | Oleanane | In vitro cytotoxicity: inhibitory effect (%) at 50 μM • PC-3: 1.81 • K562: 6.88 • HCT-15: 10.46 •MCF-7: 3.24 • SKLU-1: 21.95 Anti-inflammatory activity in mouse edema inhibition at 1 μmol/ear: 48.26% |
[41] |
| Sapium lateriflorum | 3β-palmitoxlioxi-1β,11α-dihydroxi-olean-12-ene | Oleanane | In vitro cytotoxicity: inhibitory effect (%) at 50 μM • U251: 29.56 • PC-3: 20.72 • K562: 9.86 • HCT-15: 7.24 • MCF-7: 11.53 • SKLU-1: 12.89 Anti-inflammatory activity in mouse edema inhibition at 1 μmol/ear: 68.76% |
[41] |
| lupeyl palmitate | Lupane | In vitro cytotoxicity: inhibitory effect (%) at 50 μM • PC-3: 3.05 • K562: 3.46 • HCT-15: 10.24 •MCF-7: 13.43 • SKLU-1: 19.59 Anti-inflammatory activity in mouse edema inhibition at 1 μmol/ear: 22.31% |
||
| 3β-palmitoyloxy-11-oxo- olean-12-ene | Oleanane | In vitro cytotoxicity: inhibitory effect (%) at 50 μM • PC-3: 0.08 • HCT-15: 18.69 •MCF-7: 5.15 • SKLU-1: 20.06 Anti-inflammatory activity in mouse ear edema inhibition at 1 μmol/ear: 31.49%, ID50: 0.60 μmol/ear Anti-inflammatory activity in carrageenan-induced mouse plantar edema at 31.6 mg/kg: 48.2% after 3 h |
||
| Phoradendron reichenbachianum (Viscaceae) | Ursolic acid | Ursane | Vasorelaxant effect on rat aorta: EC50 11.7 μM, Emax 72.59% |
[42] |
| Moronic acid | Oleanane | Vasorelaxant effect on rat aorta: EC50 11.7 μM, Emax 92.01% |
||
| Morolic acid | Oleanane | Vasorelaxant effect on rat aorta: EC50 94.19 μM, Emax 73.75% |
||
| Betulinic acid | Lupane | Vasorelaxant effect on rat aorta: EC50 58.46 μM, Emax 79.01% |
||
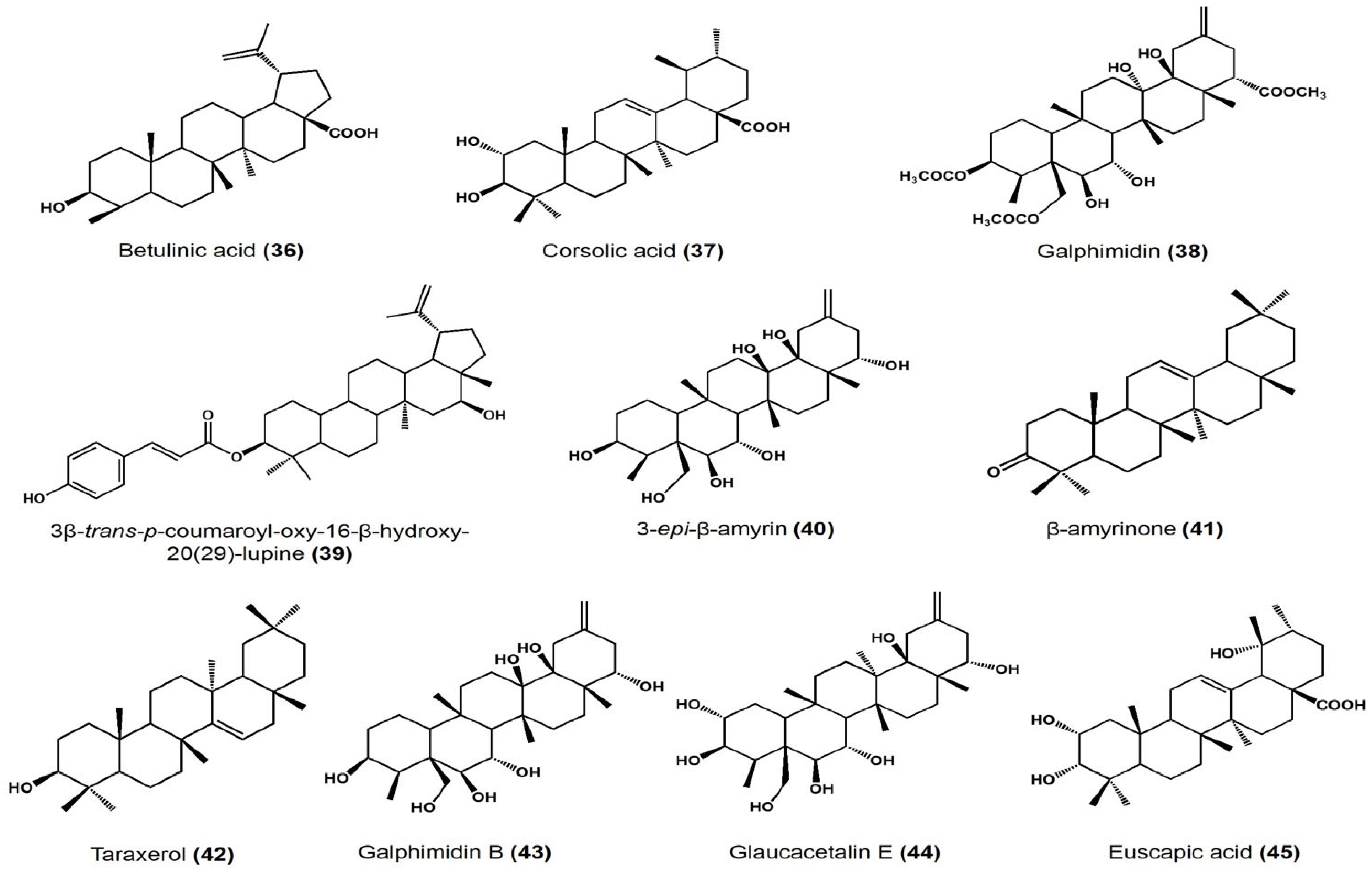
| Cratageus gracilior | Corsolic acid | Ursane | Vasodilatory effect on rat aorta: EC50 108.9 ± 6.7 μM, Emax 96.4 ± 4.2% |
[43] |
| Galphimia glauca | Galphimidin | Nor-seco friedelane | Vasodilatory effect on rat aorta: EC50 145.9 ± 6.7 μM, Emax 99.5 ± 5.3% |
|
| Jatropha neupaciflora | 3β-trans-p-coumaroyl-oxy-16-β-hydroxy-20(29)-lupene | Lupane | Vasodilatory effect on rat aorta: 63.2 ± 5.8 μM EC50 and 27.5 ± 1.9% |
|
| Sebastiania adenophora | 3-epi-β-amyrin | Ursane | Approximate effect on root growth at 250 μg/mL • Lycopersicon esculentum: −21% • Echinocloa crus-galli: −36% • Amaranthus hypochondriacus: +55% |
[44] |
| β-amyrinone | Ursane | Approximate effect on root growth at 250 μg/mL • L. esculentum: −28% • E. crus-galli: −28% • A. hypochondriacus: +39% |
||
| 3-epi-lupeol | Lupane | Approximate effect on root growth at 250 μg/mL • L. esculentum: −32% • E. crus-galli: −9% • A. hypochondriacus: +47% |
||
| Lupenone | Lupane | Approximate effect on root growth at 250 μg/mL • L. esculentum: −36% • E. crus-galli: −5% • A. hypochondriacus: +27% |
||
| Taraxerol | Taraxerane | Approximate effect on root growth at 250 μg/mL • L. esculentum: −41% • E. crus-galli: −77% • A. hypochondriacus: +39% |
||
| Taraxerone | Taraxerane | Approximate effect on root growth at 250 μg/mL • L. esculentum: −49% • E. crus-galli: −44% • A. hypochondriacus: +23% |
||
| Galphimia glauca | Glaucacetalin E | Nor-seco friedelane | Anxiolytic effect on mice at 1, 10, and 30 mg/kg dose | [45] |
| Galphimidin B | Anxiolytic effect on mice at 1, 10, and 30 mg/kg dose | |||
| Galphimidin | Anxiolytic effect on mice at 1, 10, and 30 mg/kg dose |
