Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 3 by Jessie Wu.
Root-knot nematodes (Meloidogyne spp.) are sedentary endoparasites that cause severe economic losses to agricultural crops globally. Due to the regulations of the European Union on the application of nematicides, it is crucial now to discover eco-friendly control strategies for nematode management. Biocontrol is one such safe and reliable method for managing these polyphagous nematodes. Biocontrol agents not only control these parasitic nematodes but also improve plant growth and induce systemic resistance in plants against a variety of biotic stresses. A wide range of organisms such as bacteria, fungi, viruses, and protozoans live in their natural mode as nematode antagonists.
- biocontrol
- disease
- antagonist
1. Bacteria as Biocontrol Agents against Root-Knot Nematodes
Microbial biocontrol agents exploit antagonism through hyper-parasitism and antibiosis to interfere with and inhibit the growth of another pathogen [1]. Glick [2] found that soil bacteria that promote plant growth are found in alliance with the roots/leaves/flowers or any plant tissues and are generally called plant growth-promoting bacteria (PGPB). Both the plant and microbial community can be influenced by bacteria and their products [3]. Various bacterial species have been evaluated for their nematicidal activities. Based on their mechanisms of operation, bacterial antagonists of nematodes can be grouped into rhizobacteria, obligate parasitic bacteria, endophytic bacteria, opportunistic parasitic bacteria, symbiotic bacteria, and parasporal crystal (cry) protein-forming bacteria [4]. Many bacteria such as Pseudomonas spp., Bacillus spp., Burkholderia spp., and others minimize damage in plants, as they build metabolites that then alter nematode behavior, feeding and reproduction [5]. Bacillus and Pseudomonas occur widely in the natural environment and have shown the highest efficacy for biological control [6]. In the rhizosphere, Bacillus spp. and Pseudomonas spp. are the prominent opponents of plant pathogens [7]. Pseudomonas spp. are largely present in soil and plant root systems. They can take advantage of plant exudates for nutritional purposes, produce certain metabolites and antimicrobial compounds, and can be easily produced in the laboratory [8]. Some of the important bacterial species are summarized in Table 1.
Table 1. Bacterial species as antagonists against root-knot nematodes.
| Species Name | Concentration Used | Reduction in Diseases/Result | Crop | Nematode Managed | References |
|---|---|---|---|---|---|
| Pseudomonas jessenii Verhille, et al. 1999, and Pseudomonas synxantha (Ehrenberg 1840) Holland 1920 | 25%, 50%, 75%, 100% | All concentrations greater than 75% resulted in 100% mortality of J2. | Tomato (Solanum lycopersicum L.) | Meloidogyne incognita | [9] |
| Bacillus isolates (BC 27, BC 29, and BC 31) | 108 spores mL−1 | BC 27 and BC 29 caused 100% mortality after 24 h. BC 31 was less effective compared to BC 27 and BC 29, as it caused only 84% mortality after 24 h. | Soybean (Glycine max (L.) Merr.) | Meloidogyne javanica | [10] |
[10], under in-vitro conditions, tested 70 Bacillus isolates against M. javanica J2 on soybean. With serial dilutions, primary spore suspension was settled to 108 per ml, and it was found that five isolates, BC 27, BC 29, BC 31, BC 56, and BC 64, caused mortality greater than 50%. From these five isolates, only three (BC 27, BC 29, and BC 31) from the rhizosphere of grass in goat pastures were chosen for second screening, as they caused greater larval mortality (80%) after 24 h. In a second in vitroscreening, it was found that BC 27 was remarkably better than BC 29 and BC 31, as it caused mortality of 100% after only 3 h, and BC 29 caused greater mortality than BC 31. After 24 h, both BC 27 and BC 29 were found to be more operative than BC 31 (Table 1). Under glasshouse experiments, in comparison to control, bacterial isolates BC 27 and BC 29 greatly reduced gall formation and the number of egg masses. The biocontrol potential of Bacillus subtilis, Bacillus pumilis, Bacillus thuringiensis, and Bacillus altitudinis is briefly summarized in Table 1.
Bacillus spp. have also been largely used for the effective management of plant-parasitic nematodes [15]. Bacillus spp. reduce the threat of chemical application by forming nematicidal metabolites [16][17]. Bacillus subtilis, a potential biocontrol agent, possesses spore-forming ability and several other characteristics that increases its chances of survival in the rhizosphere [18]. The genus Pasteuria, which includes the endospore-forming parasites, has also been found to decrease the populations of root-knot nematodes on various crops such as tomato, grapevines (Vitis vinifera L.), tobacco (Nicotiana tabacum L.) and peanut (Arachis hypogaea L.) [19]. It is a host-specific parasite of root-knot nematodes resistant to various nematicides and has a high level of virulence. Cetintas and Dickson [20] reported that in the presence of Pasteuria penetrans, the numbers of root galls by Meloidogyne arenaria race 1 were reduced on peanut. Similarly, Cho et al. [21] found that Meloidogyne arenaria was controlled by Pasteuria penetrans on tomato. Another bacterium, Serratia plymuthica, is found almost everywhere and can be used to control M. incognita (Table 1). It produces a large palette of antimicrobial products [3].
2. Fungi as Biocontrol Agents against Root-Knot Nematodes
Because fungi have a very high reproductive rate (both sexually and asexually), a short generation time, and are target-specific, the potential for the application of fungal biological control agents against plant pathogens has greatly expanded. Furthermore, in the absence of the host, they can survive in the environment by switching from parasitism to saprotrophism, allowing them to remain sustainable [22]. These fungi play an important role in controlling root-knot disease of plants caused by Meloidogyne spp. [23]. Different fungi have been found with nematophagous/nematicidal activities (Table 2). These fungi use different mechanisms to kill or control root-knot nematodes (Figure 1). More than 700 nematophagous fungi belonging to the phyla ascomycota, zygomycota, chytridiomycota, basidiomycota and oomycota have been described [24]. Nematophagous fungi were of four types: endoparasitic fungi, nematode-trapping fungi (predatory or scavengers), opportunistic ovicidal or parasites of eggs and females, and toxin-producing fungi [25][26].
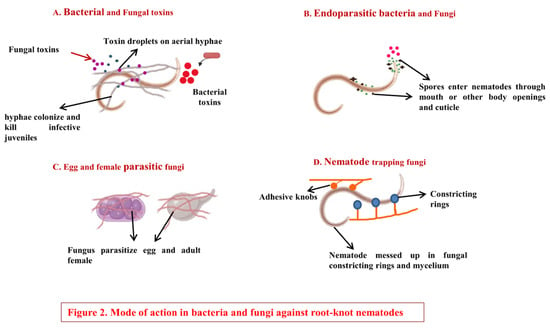
Figure 1. Mechanisms of bacterial and fungal biocontrol. (A). Bacterial and fungal toxins: Bacteria (e.g., Bacillus thuringiensis) and fungi (e.g., Pleurotus ostreatus) produce certain toxins that suppress plant-parasitic nematodes by preventing their hatching and may even cause death to juveniles [27]. (B). Endoparasitic bacteria and fungi: They produce motile spores that enter nematodes through the mouth or other body openings and cuticle and may be lethal to nematodes. (C). Egg- and female-parasitic fungi: Certain fungi such as Pochonia chlamydosporia parasitize the eggs and adult females of plant-parasitic nematodes [27]. They form branched mycelia around eggs and adult females and suppress plant-parasitic nematodes. (D). Nematode-trapping fungi: These fungi trap nematodes with the help of constricting rings or adhesive knobs and kill them, e.g., Arthrobotrys dactyloides.
Table 2. Fungal species for biocontrol of root-knot nematodes.
| Species Name | Concentration Used | Reduction in Diseases/Result | Crop | Nematode Managed | References | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Purpureocillium lilacinus Luangsa-ard, Houbraken, van Doom Hong Borman, Hywel-Jones, and Samson, 2011 | Spore suspension, 10 × 105 concentration | 85% reduction of egg masses of Meloidogyne spp. | Tomato | Meloidogyne spp. | [28] | ||||||
| Purpureocillium lilacinus | 1 × 109 cfu/mL | 76.24% reduction of root-knot diseases. | Tomato | Meloidogyne incognita | [29] | ||||||
| Pasteuria penetrans (ex Thorne 1940) Sayre and Starr 1986 | 50% spore suspension | Number of J2/100 cm | |||||||||
| Aspergillus terreus Thom 1918, Acremonium strictum W. Gams 1971 | 2% (w/w) spore load, 2.3 × 106 to 2.3 × 108 | 3 was reduced to 9.2 in soil compared to 16.6 in control. | 76% and 73% reduction in eggs/egg mass and the number of hatching egg/egg mass by A. Terrus, and 71% and 68% by A. Strictum, respectively.Babchi (Psoralea corylifolia L.) | Meloidogyne incognita | Tomato[11] | ||||||
| Meloidogyne incognita | [ | 30 | ] | Pseudomonas fluorescens (pf1) (Flugge 1886) Migula, 1895 |
107–10 | ||||||
| Acremonium implicatum (J.C. Gilman and E.V. Abbott) W. Gams, 1975 | 9 CFU/mL | Spore suspension, 1 × 10669.8% reduction of Meloidogyne incognita | CFU/mLCowpea (Vigna unguiculata (L.) Walp.) | Meloidogyne incognita | [12] | ||||||
| Reduction in galls with 40.6 galls/treated plant as compared with 121.6 on control plant. | Tomato | Meloidogyne incognita | [ | 31 | Bacillus subtilis (bs2) (Ehrenberg 1835) Cohn 1872 | 107–109 CFU/mL | 82% reduction of total nematode population | Cowpea | Meloidogyne incognita | [12] | |
| ] | |||||||||||
| Acremonium implicatum | 1 × 106 CFU/mL conidial suspension | Reduction of 60% root galls | Tomato | Meloidogyne incognita | [32] | Bacillus pumilis (bp2) Meyer and Gottheli 1901 | 107–109 CFU/mL | 81.8% reduction of nematode population | Cowpea | Meloidogyne incognita | |
| Acremonium strictum, Aspergillus niger van Tieghem 1867, Purpureocillium lilacinus and Trichoderma harzianum Rifai 1969 | [ | Talc-based formulation with spore load 2 × 108 CFU | 12 | ] | |||||||
| Combined effect of | Trichoderma harzianum | and Acremonium strictum resulted in greater reduction of disease and high yield. | Tomato | Meloidogyne incognita | [33] | Bacillus thuringiensis Berliner 1915 | 108 CFU/mL/2 | 80.5% reduction of root-knot nematode | Tomato | Meloidogyne incognita | [13] |
| Arthrobotrys dactyloides Drechsler 1937 | 2 g (10,000 spore concentration) + 1 g yeast + 3 g vermiculite + 1 mL molasses | Reduction of 94.1% of root galls per plant | Snap bean (Phaseolus vulgaris L.) | Meloidogyne incognita | [34] | Bacillus altitudinis (AMCC1040) Shivaji et al. 2006 | 108 CFU/mL | Numbers of J2s in roots and soil were reduced by 93.68% and 84.48%, respectively. | Ginger (Zingiber officinale Rosc.) | Meloidogyne incognita | [14] |
| Arthrobotrys oligospora | Pseudomonas protegens Ramette et al. 2011 | 1 × 109 | Mortality rate of 87.76% was observed in J2 24 h after treatment (in-vitro), and Gall index was reduced to 30.67% compared to 49.33% in control, and biocontrol efficacy of 37.84 was observed | Tomato | Meloidogyne incognita | [3] | |||||
| ] | Serratia plymuthica (Lehmann and Neumann 1896) Breed et al. 1948 | 1 × 109 | Mortality rate of 92.67% was observed in J2 24 h after treatment (in-vitro), and Gall index lowered to 38.67% compared to 49.33% in control, and biocontrol efficacy of 21.62% was observed | Tomato | M. incognita | [3] |
Sharma et al. [9] studied the control of root-knot disease with pseudomonad rhizobacteria filtrate under in-vitro and greenhouse conditions. Pseudomonas jessenii strain R62 and Pseudomonas synxantha strain R81 were used to control root-knot nematode (Meloidogyne incognita) on tomato plants. In laboratory conditions, it was found that out of all of the treatments (25%, 50%, 75%, 100%) with R62 and R81, 75%, 100% and all dilutions of R62 + R81 caused 100% mortality of second-stage juveniles (J2). At 25%, no effect was found on J2, and at 50% some mortality was observed. Under greenhouse conditions, by using R62 and R81 collectively, significant variations in plant growth parameters were observed. When the same treatment (R62 + R81) was given to plants under nematode stress, a great increase in plant growth parameters was found in contrast to only nematode-inoculated plants (Table 1). So, these observations indicated that Pseudomonas culture filtrate can operate as a potential biocontrol agent for controlling root-knot nematodes. Similarly, the biocontrol efficiency of Pseudomonas fluorescens and Pseudomonas protegens Sneb 1997 is summed up in Table 1.
Chinheya et al.
| Fresen 1850 |
| Spore suspension with 10 | |||||||||||
| 5 | |||||||||||
| conidia/mL | |||||||||||
| Reduced number of galls, females and nematodes and enhanced plant growth. | |||||||||||
| Tomato | |||||||||||
| Meloidogyne incognita | [ | 35 | ] | ||||||||
| Arthrobotrys oligospora | Fungal suspension at 10, 30 and 50 mL/plant (1 × 104 spore/mL) | 50 mL/plant resulted in reduction of females, eggs/egg-mass and no. of J2 in soil. | Tomato | Meloidogyne incognita | [36] | ||||||
| Arthrobotrys oligospora | 106 spores/mL | Application of fungus with salicylic acid reduced root galls and nematode population and increased plant growth. | Tomato | Meloidogyne javanica | [37] | ||||||
| Hedysarum coronarium L. | Fabaceae | Leaves, flower | Saponins, flavonoids and tannins | M. incognita | [74] | Aspergillus awamori Nakaz | 108 CFU/mL | Resulted in 44.9% reduction of nematode infection. | Tomato | ||
| Allium sativum L. | M. incognita | Alliaceae | Bulb, leaves | [ | 38] | ||||||
| Organosulfur compound, Allicin | Meloidogyne | spp. | [ | 75 | ][76] | Aspergillus japonicus ZW1 Saito 1906 | 20% fermentation broth | Resulted in 51.8 and 47.3% reduction of eggs and galls, respectively. | |||
| Cymbopogon martini (Roxb.) Watsand C. flexuosus (Nees ex Steud) W. Watson | Tomato | M. incognita | [ | 39 | ] | ||||||
| Poaceae | Leaves | Eugenol and citral | M. incognita | [ | 77] | Aspergillus welwitschiae AW2017 (Bres.) Henn. | 2 × 108 conidia/mL (5× AW2017) | Reduction by 40.5% and 24.5% of root galls and juveniles, respectively. | Rice (Oryza sativa L.) | M. graminicola | [ |
| Brassica spp. | 40 | ] | |||||||||
| Brassicaceae | Shoot, roots, seed | Isothiocynates | Meloidogyne | spp. | [78] | Fusarium and Trichoderma isolates | 5 × 106 conidial suspension per pot | 29–42% of root galling was reduced by application of conidia of rhizosphere Fusarium isolates and 38% reduction of root galls by treatment with Trichoderma. | Rice | M. graminicola | [ |
| Pistacia lentiscus L. | 41 | ] | |||||||||
| Anacardiaceae | Leaves | Quercetin, quinic and gallic acid | M. javanica | [ | 79 | Chaetomium globosum Kunze 1817 | 30 mg ChA/kg soil (Chaetoglobosin A-ChA) |
Resulted in reduction of 63% of eggs per plant | Cucumber (Cucumis sativus L.) | M. incognita | [42] |
| Chaetomium globosum YSC5 | 200 μg/Ml of chaetoglobosin B and chaetoglobosin A | 59.0–61.5% reduction in number of galls and 71.1–72.4% reduction in number of egg masses | Tomato | M. javanica | [43] | ||||||
| Dactylaria brochopaga Drechsler 1937 & Verticilium chlamydosporium Goddard 1913 | 2 g (Dactylaria + Verticilium chlamydosporium) + 3 g (vermiculite) + 1 mL (molasses) + 1 g yeast | 93.1% reduction of root galls per plant | Eggplant (Solanum melongena L.) | M. incognita | [44] | ||||||
| Dactylaria brochopaga | 2 g (fungus) + 1 g (yeast) + 1 mL (molasses) + 3 gm (vermiculite) |
Resulted in 94.1% mean reduction in the number of root galls | Cucumber | M. incognita | [45] | ||||||
| ] | Pleurotus ostreatus (Jacq.) P. Kumm. 1871 | 5, 10, 15 g fresh mashed mushroom | 15 g mushroom residue resulted in an 86.4% reduction of nematode reproduction and gall reduction by 92.4%. | cowpea | M. incognita | [46] | |||||
| Gliocladium spp. | 104 mL−1, 105 mL−1, 10−6 mL−1 conidia suspension | 106 mL−1 conidia suspension significantly decreased intensity of damage by 33%. | Tomato | Meloidogyne spp. | [47] | ||||||
| Lecanicillium muscarium R. Zare and W. Gams 2001 | 103, 104, 105 and 106 conidia levels with different inoculum densities of M. incognita (500, 1000, 1500, 2000) | Higher density 1 × 106 decreased nematode population, and plant growth parameters improved with increasing fungus inoculum. | Tomato | M. incognita | [48] | ||||||
| Purpureocillium lilacinus | P. lilacinus WP 1.15% (1 × 108 CFU/g), P. lilacinus liquid 1.50% (1 × 109 CFU/mL) and P. lilacinus AS 1.0% (2 × 106 CFU/g) | P. lilacinus liquid 1.50% resulted in 48.72% reduction in average root gall index; average number of egg masses per root system and average soil nematode population reduced by 60.15% and 61.10%, respectively. | Capsicum (Capsicum annuum L.) | M. incognita | [49] | ||||||
| Beauveria bassiana (Bals. Criv.) Vuill. 1912 | 1×, 5×, 10×, 20×, 50× dilution of culture filtrate | Resulted in 98.61% and 76.39% rates of inhibition of nematodes at 1× and 5× solutions | Tomato | M. hapla | [50] | ||||||
| Arthrobotrys dactyloides Drechsler 1937 | 4 × 106 CFU/kg of soil | Resulted in reduction of 37.9–81.8% of juveniles and 44.5–51.3% of egg masses | Tomato | M. incognita | [51] |
3. Plant Extracts as Biocontrol Agents against Root-Knot Nematodes
The richest source of organic matter on Earth is found in plants, which are repositories of nature. These days, plants and their by-products are given more consideration as biocontrol agents against a variety of plant parasites, nematodes, fungi, and other pests [52]. According to current patterns in the application of botanical nematicides, a majority of the allelopathic substances demonstrate egg hatching inhibition, interrupt sexual selection, and decrease gut motility. Nematicidal plant extracts are mostly from the families Meliaceae, Fabaceae, Lamiaceae, Brassicaceae, Verbenance, Euphorbiaceae, etc. [53]. Various secondary metabolites released from plants such as alkaloids, flavonoids, glucosinolates, isothiocyanates, tannins, fatty acids, and sesquiterpenes show nematicidal potential against egg hatching, juvenile mortality, and penetration of nematodes [54][55]. Orisajo and Dongo [56] studied the nematicidal potential of various plant extracts of Ocimum gratisimum L., Carica papaya L., Vernonia amygdalina Delile, Bixa orellana L., and Azadirachta indica A. Jusson against the reproduction and pathogenicity of Meloidogyne incognita. Fabiyi [57] used plant materials of Eucalyptus officinalis, Ocimum gratismum, Hyptis suaveolens (L.) Kuntze and Crotolaria juncea L. as soil amendments to control M. incognita on okra. Cucurbitaceae cold peeling extracts (CCOPEs) obtained from the peels of Cucumis melo L. var. cantalupensis protected Oryza sativa L. and Solanum lycopersicum L. against root-knot nematodes by direct nematicidal effects and through resistance induced by the generation of reactive oxygen species (ROS) and ethylene accumulation and cell wall modification [58]. Arshad et al. [59] found that seed priming with botanical extracts, Neem (Azadirachta indica), Datura (Datura stramonium L.), Kortuma (Citrullus colocynthis (L.) Schrad) and Moringa oleifera Lam. leaf extracts showed significant results in suppressing nematodes. In-vitro neem leaf extract resulted in egg hatching reduction by 30% and juvenile mortality of 70%, and in pot trials, significantly reduced the numbers of galls, egg masses, and females and increased juvenile mortality. An aqueous extract of Phyllanthus amarus Schumach. and Thonn. (5000 ppm) resulted in 91% mortality of juveniles after 72 h of exposure and 86.5% reduction in egg hatching after 7 days of exposure [60]. Different plant parts, including by-products such as oil cakes, chopped leaves as soil or organic amendments, and plant extracts for soil drenching, seed dressing and priming have been assessed for their nematicidal properties: specifically, rhizome of Curcuma longa L., aerial parts of Lantana camara L., Azadirachta indica, Datura, and roots of Fumaria parviflora Lam., bark of Terminalia nigrovenulosa Pierre, 1886, and bulbs of Allium sativum L. Table 3 summarizes a few of the plant species, their secondary metabolites, and nematodes targeted.
Table 3. Plant extracts for biocontrol of root-knot nematodes.
| Plant Species | Family | Plant Parts Used | Active Compounds | Nematodes Targeted | References |
|---|---|---|---|---|---|
| Azadirachta indica | Meliaceae | Leaf, Seed, Fruit, Root, Bark | Azadirachtin | Meloidogyne incognita, M. javanica | [61][62] |
| Lantana camara | Verbenaceae | Aerial part | Lantanilic acid, camaric acid and oleanolic acid | M. incognita | [63] |
| Fumaria parviflora | Papaveraceae | Root | Nonacosane-10-ol and 23a-homostigmast-5-en-3β-ol | M. incognita | [64] |
| Tageteserecta L. | Asteraceae | Leaves | Alpha-terthienyl | M. incognita, M. javanica | [65][66][67] |
| Moringa oleifera | Moringaceae | Leaves | Flavonoids, glycosides, saponin | M. incognita | [68] |
| Terminalia nigrovenulosa | Combretaceae | Bark | 3,4-dihydroxybenzoic acid (3,4-DHBA) | M. incognita | [69] |
| Datura spp. | Solanaceae | Leaves, inflorescence, roots |
Atropine, scopolamine, hyoscyamine | Meloidogyne spp. | [70] |
| Juglans regia L. | Juglandaceae | Leaves, husk | Beta 1, 4 naphthoquinones | M. hispanica, M. luci | [71][72] |
| Waltheria indica L. | Malvaceae | Roots | 5-methoxywaltherione A, waltherione A and waltherione C | M. incognita, M. hapla, M. arenaria | [73 |
References
- Miliute, I.; Buzaite, O.; Baniulis, D.; Stanys, V. Bacterial endophytes in agricultural crops and their role in stress tolerance: A review. Zemdirbyste 2015, 102, 465–478.
- Glick, B.R. Beneficial Plant-Bacterial Interactions; Springer: Berlin/Heidelberg, Germany, 2015; pp. 139–180.
- Zhao, J.; Wang, S.; Zhu, X.; Wang, Y.; Liu, X.; Duan, Y.; Chen, L. Isolation, and characterization of nodules endophytic bacteria Pseudomonas protegens Sneb 1997and Serratia plymuthica Sneb 2001 for the biological control of root-knot nematode. Appl. Soil Ecol. 2021, 164, 103924.
- Tian, B.; Yang, J.; Zhang, K.Q. Bacteria used in the biological control of plant-parasitic nematodes: Populations, mechanisms of action, and future prospects. FEMS Microbiol. Ecol. 2007, 61, 197–213.
- Tariq, M.; Khan, A.; Asif, M.; Khan, F.; Ansari, T.; Shariq, M.; Siddiqui, M.A. Biological control: A sustainable and practical approach for plant disease management. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2020, 70, 507–524.
- Migunova, V.D.; Sasanelli, N. Bacteria as biocontrol tool against phytoparasitic nematodes. Plants 2021, 10, 389.
- Mhatre, P.H.; Karthik, C.; Kadirvelu, K.; Divya, K.L.; Venkatasalam, E.P.; Srinivasan, S.; Shanmuganathan, R. Plant growth promoting rhizobacteria(PGPR): A potential alternative tool for nematodes biocontrol. Biocatal. Agric. Biotechnol. 2019, 17, 119–128.
- Panpatte, D.G.; Jhala, Y.K.; Shelat, H.N.; Vyas, R.V. Pseudomonas fluorescens: A promising biocontrol agent and PGPR for sustainable agriculture. In Microbial Inoculants in Sustainable Agricultural Productivity; Springer: New Delhi, India, 2016; pp. 257–270.
- Sharma, I.P.; Sharma, A.K. Effective control of root-knot nematode disease with Pseudomonad rhizobacteria filtrate. Rhizosphere 2017, 3, 123–125.
- Chinheya, C.C.; Yobo, K.S.; Laing, M.D. Biological control of the root-knot nematode, Meloidogyne javanica (Chitwood) using Bacillus isolates, on soybean. Biol. Control 2017, 109, 37–41.
- Mehtab, A.; Javed, N.; Khan, S.A.; Gondal, A.S. Combined effect of Pasteuria penetrans and neem extract on the development of root-knot nematode in medicinal plants. Pak. J. Nematol. 2013, 31, 55–59.
- Abd-El-Khair, H.; El-Nagdi, W.; Youssef, M.; Abd-Elgawad, M.M.; Dawood, M.G. Protective effect of Bacillus subtilis, B. pumilus, and Pseudomonas fluorescens isolates against root-knot nematode Meloidogyne incognita on cowpea. Bull. Natl. Res. Cent. 2019, 43, 1–7.
- Khalil, M.S.; Kenawy, A.; Gohrab, M.A.; Mohammed, E.E. Impact of microbial agents on Meloidogyne incognita management and morphogenesis of tomato. J. Biopestic. 2012, 5, 28–35.
- Wang, J.Y.; Guo, C.; Zhao, P.; Yu, F.Y.; Su, Y.; Qu, J.P.; Zhou, B. Biocontrol potential of Bacillus altitudinis AMCC1040 against root-knot nematode disease of ginger and its impact on rhizosphere microbial community. Biol. Control 2021, 158, 104598.
- Lee, Y.S.; Kim, K.Y. Antagonistic potential of Bacillus pumilus L1 against root-knot nematode(Meloidogyne arenaria). J. Phytopathol. 2016, 164, 29–39.
- Abbasi, M.W.; Ahmed, N.; Zaki, M.J.; Shuakat, S.S.; Khan, D. Potential of Bacillus species against Meloidogyne javanica parasitizing eggplant (Solanum melongena L.) and induced biochemical changes. Plant Soil 2014, 375, 159–173.
- Sivasakthi, S.; Usharani, G.; Saranraj, P. Biocontrol potentiality of plant growth promoting bacteria (PGPR)-Pseudomonas fluorescens and Bacillus subtilis: A review. Afr. J. Agric. Res. 2014, 9, 1265–1277.
- Rao, M.S.; Kamalnath, M.; Umamaheswari, R.; Rajinikanth, R.; Prabu, P.; Priti, K.; Gopalakrishnan, C. Bacillus subtilis IIHRBS-2 enriched vermicompost controls root-knot nematode and soft rot disease complex in carrot. Sci. Hortic. 2017, 218, 56–62.
- Bhuiyan, S.A.; Garlick, K.; Anderson, J.M.; Wickramasinghe, P.; Stirling, G.R. Biological control of root-knot nematode on sugarcane in soil naturally or artificially infested with Pasteuria penetrans. Australas. Plant Pathol. 2018, 47, 45–52.
- Cetintas, R.; Dickson, D.W. Persistence and Suppressiveness of Pasteuria penetrans to Meloidogyne arenaria Race. J. Nematol. 2004, 36, 540.
- Cho, M.R.; Na, S.Y.; Yiem, M.S. Biological control of Meloidogyne arenaria by Pasteuria penetrans. J. Asia-Pac. Entomol. 2000, 3, 71–76.
- Thambugala, K.M.; Daranagama, D.A.; Phillips, A.J.; Kannangara, S.D.; Promputtha, I. Fungi vs. fungi in biocontrol: An overview of fungal antagonists applied against fungal plant pathogens. Front. Cell. Infect. Microbiol. 2020, 10, 604923.
- Stirling, G.R.; West, L.M. Fungal parasites of root-knot nematode eggs from tropical and subtropical regions of Australia. Australas. Plant Pathol. 1991, 20, 149–154.
- Li, J.; Zou, C.; Xu, J.; Ji, X.; Niu, X.; Yang, J.; Zhang, K.Q. Molecular mechanisms of nematode-nematophagous microbe interactions: Basis for biological control of plant-parasitic nematodes. Annu. Rev. Phytopathol. 2015, 53, 67–95.
- Li, Y.; Zhang, C.; Yin, Y.; Cui, F.; Cai, J.; Chen, Z.; Hu, R. Neurological effects of pesticide use among farmers in China. Int. J. Environ. Res. Public Health 2014, 11, 3995–4006.
- Zhang, Y.; Yang, G.; Fang, M.; Deng, C.; Zhang, K.Q.; Yu, Z.; Xu, J. Comparative analyses of mitochondrial genomes provide evolutionary insights into nematode-trapping fungi. Front. Microbiol. 2020, 11, 617.
- Abd Elgawad, M.M.; Askary, T.H. Fungal and bacterial nematicides in integrated nematode management strategies. Egypt. J. Biol. Pest Control 2018, 28, 1–24.
- Ahmed, S.; Monjil, M.S. Effect of Paecilomyceslilacinus ontomatoplantsandthemanagement of root-knot nematodes: Paecilomyces lilacinus on tomato root-knot disease. J. Bangladesh Agric. Univ. 2019, 17, 9–13.
- Khalil, M.S.E.D.H.; Allam, A.F.G.; Barakat, A.S.T. Nematicidal activity of some biopesticide agents and microorganisms against root-knot nematode on tomato plants under green houseconditions. J. Plant Prot. Res. 2012, 52, 47–52.
- Singh, S.; Mathur, N. Biological control of root-knot nematode (Meloidogyne incognita) infesting tomato. Biocontrol Sci. Technol. 2010, 20, 865–874.
- Tian, X.; Yao, Y.; Chen, G.; Mao, Z.; Wang, X.; Xie, B. Suppression of Meloidogyne incognita by the endophytic fungus Acremonium implicatum from tomato root galls. Int. J. Pest Manag. 2014, 60, 239–245.
- Yao, Y.R.; Tian, X.L.; S hen, B.M.; Mao, Z.C.; Chen, G.H.; Xie, B.Y. Transformation of the endophytic fungus Acremonium implicatum with GFP and evaluation of its biocontrol effect against Meloidogyne incognita. World J. Microbiol. Biotechnol. 2015, 31, 549–556.
- Goswami, J.; Pandey, R.K.; Tewari, J.P.; Goswami, B.K. Management of root-knot nematode on tomato through application of fungal antagonists, Acremonium strictum and Trichoderma harzianum. J. Environ. Sci. Health Part B 2008, 43, 237–240.
- Noweer, E.M.A. A field trial to use the nematode-trapping fungus (Arthrobotrys dactyloides) to control the root-knot nematode(Meloidogyne incognita) infesting bean plants. Comm. Appl. Biol. Sci. Ghent. Univ. 2017, 82, 275–280.
- Soliman, M.S.; El-Deriny, M.M.; Ibrahim, D.S.S.; Zakaria, H.; Ahmed, Y. Suppression of root-knot nematode Meloidogyne incognita on tomato plants using the nematode trapping fungus Arthrobotrys oligospora Fresenius. J. Appl. Microbiol. 2021, 131, 2402–2415.
- Bakr, R.A.; Mahdy, M.E.; Mousa, E.S.M. Biological control of root-knot nematode (Meloidogyne incognita) by Arthrobotrys oligospora. Egypt. J. Crop Prot. 2014, 9, 1–11.
- Mostafanezhad, H.; Sahebani, N.; Nourinejhad Zarghani, S. Control of root-knot nematode (Meloidogyne javanica) with combination of Arthrobotrys oligospora and salicylic acid and study of some plant defence responses. Biocontrol. Sci. Technol. 2014, 24, 203–215.
- Cui, R.; Fan, C.; Sun, X. Isolation, and characterisation of Aspergillus awamori BS05, a root-knot nematode trapping fungus. Biocontrol. Sci. Technol. 2015, 25, 1233–1240.
- He, Q.; Wang, D.; Li, B.; Maqsood, A.; Wu, H. Nematicidal evaluation and active compounds isolation of Aspergillus japonicus ZW1 against root-knot nematodes (Meloidogyne incognita). Agronomy 2020, 10, 1222.
- Ying, L.I.U.; Zhong, D.; Peng, D.L.; Liu, S.M.; Kong, L.A.; Huan, P.; Huang, W.K. Evaluation of the biocontrol potential of Aspergillus welwitschiae against the root-knot nematode (Meloidogyne graminicola)in rice(Oryza sativa L.). J. Integr. Agric. 2019, 18, 2561–2570.
- Le, H.T.; Padgham, J.L.; Sikora, R.A. Biological control of the rice root-knot nematode (Meloidogyne graminicola) on rice, using endophytic and rhizosphere fungi. Int. J. Pest Manag. 2009, 55, 31–36.
- Hu, Y.; Zhang, W.; Zhang, P.; Ruan, W.; Zhu, X. Nematicidal activity of chaetoglobosin A produced by Chaetomium globosum NK102 against Meloidogyne incognita. J. Agric. Food Chem. 2013, 61, 41–46.
- Khan, B.; Yan, W.; Wei, S.; Wang, Z.; Zhao, S.; Cao, L.; Ye, Y. Nematicidal metabolites from endophytic fungus (Chaetomium globosum YSC5). FEMS Microbiol. Lett. 2019, 366, fnz169.
- Noweer, E.M.A.; Elkelany, U.S. Biological control of root-knot nematode (Meloidogyne incognita) infesting eggplant by the nematode-trapping fungus (Dactylaria brochopaga) and the nematode egg parasitic fungus (Verticilium chlamydosporium) under field conditions. J. Innov. Pharm. Biol. Sci. 2019, 6, 1–6.
- Noweer, E.M.A.; Aboul-Eid, H.Z. Biological control of root-knot nematode Meloidogyne incognita infesting cucumber Cucumis sativus L. cvs. Alfa by the nematode-trapping fungus (Dactylaria brochopaga) under field conditions. Agric. Biol. J. North Am. 2013, 4, 435–440.
- Youssef, M.M.; El-Nagdi, W.M. New approach for biocontrolling root-knot nematode (Meloidogyne incognita) on cowpea by commercial fresh oyster mushroom (Pleurotus ostreatus). Jordan J. Biol. Sci. 2021, 14, 173–177.
- Amin, N. The use of fungal endophytes Gliocladium spp. In different concentration to control of root-knot nematode(Meloidogyne spp.). Acad. Res. Int. 2014, 5, 91.
- Hussain, M.; Zouhar, M.; Rysanek, P. Population dynamics of a nematophagous fungus Lecanicillium muscarium, and root-knot nematode (Meloidogyne incognita) to assess the disease pressure and its management. Pak. J. Zool. 2017, 49, 197–204.
- Bawa, N.; Kaur, S.; Dhillon, N.K. Integrated management of root-knot nematode (M. incognita) in capsicum, using Paecilomyces lilacinus and organic amendments. J. Entomol. Zool. Stud. 2020, 8, 1693–1701.
- Liu, T.; Wang, L.; Duan, Y.X.; Wang, X. Nematicidal activity of culture filtrate of Beauveria bassiana against Meloidogyne hapla. World J. Microbiol. Biotechnol. 2008, 24, 113–118.
- Kumar, D.; Singh, K.P. Assessment of predacity and efficacy of Arthrobotrys dactyloides for biological control of root-knot disease of tomato. J. Phytopathol. 2006, 154, 1–5.
- Grainge, M.; Ahmed, S. Handbook of Plants with Pest-Control Properties; John Wiley & Sons Limited: Hoboken, NJ, USA, 1988; p. 470.
- Mwamula, A.O.; Kabir, M.F.; Lee, D. A Review of the Potency of Plant Extracts and Compounds from Key Families as an Alternative to Synthetic Nematicides: History, Efficacy, and Current Developments. Plant Pathol. J. 2022, 38, 53–77.
- Gommers, F.J. Biochemical interactions between nematodes and plants and the irrelevance to control. Helminthol. Abstr. 1981, 50, 9–24.
- Chitwood, D.J. Phytochemical based strategies for nematode control. Annu. Rev. Phytopathol. 2002, 40, 221–249.
- Orisajo, S.B.; Dongo, L.N. Nematicidal potential of some indigenous plant extracts against root-knot nematode on cacao. Afr. Sci. 2022, 6, 129–134.
- Fabiyi, O.A. Evaluation of plant materials as root-knot nematode (Meloidogyne incognita) suppressant in okra(Abelmoschus esculentus). Agric. Conspec. Sci. 2021, 86, 51–56.
- De Kesel, J.; Degroote, E.; Nkurunziza, R.; Singh, R.R.; Demeestere, K.; DeKock, K.; Kyndt, T. Cucurbitaceae cold Peeling Extracts (CCOPEs) protect plants from root-knot nematode infections through induced resistance and nematicidal effects. Front. Plant Sci. 2022, 12, 785699.
- Arshad, U.; Jabran, M.; Ahmed, S.; Abbas, A.; Jabbar, A.; Zahid, M.S.; Ali, M.A. Seed-Priming: A novel approach for improving growth performance and resistance against root-knot nematode (Meloidogyne incognita) in bread wheat(Triticum aestivum L.). Gesunde Pflanzen. 2022, 74, 1041–1051.
- Khan, F.; Asif, M.; Khan, A.; Tariq, M.; Ansari, T.; Shariq, M.; Siddiqui, M.A. Evaluation of the nematicidal potential of some botanicals against root-knot nematode, Meloidogyne incognita infected carrot: In in-vitro and greenhouse study. Curr. Plant Biol. 2019, 20, 100115.
- Lynn, O.M.; Song, W.G.; Shim, J.K.; Kim, J.E.; Lee, K.Y. Effects of azadirachtin and neem-based formulations for the control of sweet potato whitefly and root-knot nematode. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 598–604.
- Javed, N.; Gowen, S.R.; El-Hassan, S.A.; Inam-ul-Haq, M.; Shahina, F.; Pembroke, B. Efficacy of neem (Azadirachta indica) formulations on biology of root-knot nematodes (Meloidogyne javanica) on tomato. Crop Prot. 2008, 27, 36–43.
- Qamar, F.; Begum, S.; Raza, S.M.; Wahab, A.; Siddiqui, B.S. Nematicidal natural products from the aerial parts of Lantana camara Linn. Nat. Prod. Res. 2005, 19, 609–613.
- Naz, I.; Khan, M.R. Nematicidal activity of nonacosane-10-ol and 23a-homostigmast-5-en-3β-ol isolated from the roots of Fumaria parviflora (Fumariaceae). J. Agric. Food Chem. 2013, 61, 5689–5695.
- Adaka, P.; Singh, A.; Dhiman, P.; Chandrika, K.P.; Walia, S.; Sirohi, A.; Parmar, B.S. Hydrogel based formulations of Tagetes patula root extract and MgSO4 to control Meloidogyne incognita in cucumber. Allelopathy J. 2017, 40, 173–186.
- Das, S.; Wadud, A.; Khokon, M.A.R. Evaluation of the effect of different concentrations of organic amendments and botanical extracts on the mortality and hatching of Meloidogyne javanica. Saudi J. Biol. Sci. 2021, 28, 3759–3767.
- Arshad, U.; Butt, H.; Ali, M.A.; Jabran, M.; Zahid, M.S.; Sarfraz, S. Exploring the nematicidal activity of plant extracts for management of Meloidogyne incognita in local cultivars of eggplant (Solanum melongena L.) in Pakistan. Arch. Phytopathol. Plant Prot. 2021, 54, 2333–2344.
- Khairy, D.; Refaei, A.; Mostafa, F. Management of Meloidogyne incognita infecting eggplant using Moringa extracts, vermicompost, and two commercial bio-products. Egypt. J. Agronematology 2021, 20, 1–16.
- Seo, D.J.; Kim, K.Y.; Park, R.D.; Kim, D.H.; Han, Y.S.; Kim, T.H.; Jung, W.J. Nematicidal activity of 3,4-dihydroxy benzoic acid purified from Terminalia nigrovenulosa bark against Meloidogyne incognita. Microb. Pathog. 2013, 59, 52–59.
- Bakr, R.A. Nematicidal activity of Jimson weed (Datura spp.) for management of plant-parasitic nematodes with emphasis on root-knot nematode: A review. Pak. J. Phytopathol. 2021, 33, 183–204.
- Maleita, C.; Esteves, I.; Braga, M.E.; Figueiredo, J.; Gaspar, M.C.; Abrantes, I.; de Sousa, H.C. Juglone and 1,4-Naphthoquinone promising nematicides for sustainable control of the root-knot nematode Meloidogyne luci. Front. Plant Sci. 2022, 13, 867803.
- Maleita, C.; Esteves, I.; Chim, R.; Fonseca, L.; Braga, M.E.; Abrantes, I.; de Sousa, H.C. Naphthoquinones from walnut husk residues show strong nematicidal activities against the root-knot nematode Meloidogyne hispanica. ACS Sustain. Chem. Engineering 2017, 5, 3390–3398.
- Jang, J.; Le Dang, Q.; Choi, G.J.; Park, H.W.; Kim, J.C. Control of root-knot nematodes using Waltheria indica producing 4-quinolone alkaloids. Pest Manag. Sci. 2019, 75, 2264–2270.
- D’Addabbo, T.; Tava, A.; Argentieri, M.P.; Biazzi, E.; Candido, V.; Avato, P. Nematicidal Potential of Sulla (Hedysarum coronarium, L.) against the root-knot nematode Meloidogyne incognita. Plants 2022, 11, 2550.
- Khairan, K.; Yusra, N.; Eriana, C.N.; Bahi, M.; Syaukani, S.; Sriwati, R.; Jacob, C. Termiticidal and Nematicidal activities of five extracts from Garlic (Allium sativum). In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2021; Volume 1882, No.1; p. 012121.
- Jacob, C. A scent of therapy: Pharmacological implications of natural products containing redox-active sulphur atoms. Nat. Prod. Rep. 2006, 23, 851–863.
- Ajith, M.; Pankaj; Shakil, N.A.; Kaushik, P.; Rana, V.S. Chemical composition and nematicidal activity of essential oils and their major compounds against Meloidogyne graminicola (rice root-knot nematode). J. Essent. Oil Res. 2020, 32, 526–535.
- Ntalli, N.; Caboni, P. A review of isothiocyanates biofumigation activity on plant-parasitic nematodes. Phytochem. Rev. 2017, 16, 827–834.
- Hajji-Hedfi, L.; Larayedh, A.; Hammas, N.C.; Regaieg, H.; Horrigue-Raouani, N. Biological activities and chemical composition of Pistacia lentiscus in controlling Fusarium wilt and root-knot nematode disease complex on tomato. Eur. J. Plant Pathol. 2019, 155, 281–291.
More
