Maternal anti-Ro antibodies present during the fetal period can cause complications ranging from congenital heart block, fetal demise or long-term consequences.
The anti-Ro induced autoimmune reaction causes long-term fibrosis and calcification of the conductive tissue. In addition, the CHB injury mechanisms were shown to involve other factors, like fetal susceptibility that increases for every subsequent pregnancy, from a 2% incidence in the case of nulliparous mothers. The predictive value of maternal anti-Ro antibodies for CHB high-risk pregnancy is low, and other markers are lacking, making this condition difficult to efficiently monitor. Moreover, the positive anti-Ro pregnancies do not benefit from a prophylactic treatment or from an efficient therapy once CHB was diagnosed. Thus, new data from ongoing trials are highly expected, to provide both potential biomarkers and therapeutic solutions.
This entry illustrates the current understanding of the anti-Ro antibodies associated pathologies, from the perspective of specialists involved in its management, emphasizing key issues, missing links, and possible future directions for an effective interdisciplinary approach.
- Anti Ro antibodies
- congenital heart block
- neonatal lupus
1. Introduction
The presence of maternal Anti-Ro/Anti-La antibodies causes a passively acquired autoimmunity that may be associated with serious fetal complications. The classic example is the autoimmune-mediated congenital heart block (CHB) which is due in most cases to the transplacental passage of Anti-Ro/Anti-La antibodies. The exact mechanisms through which these pathologic events arise are linked to disturbances in calcium channels function, impairment of calcium homeostasis and ultimately apoptosis, inflammation and fibrosis. CHB still represents a challenging diagnosis and a source of debate regarding the best management. As the third-degree block is usually irreversible, the best strategy is risk awareness and prevention. Although CHB is a rare occurrence, it affects one in 20,000 live births, with a high overall mortality rate (up to 20%, with 70% of in utero deaths).
2. Pregnancy in Women with Anti-Ro/SSA Antibodies
2.1. Frequency of Gestational Complications
The risk of developing a congenital fetal heart block varies between 0.2–2% in nulliparous women with positive anti-Ro antibodies, and increases to 15–20% in pregnancies with a previously affected fetus or neonate [25,32][1][2]. Women who had two previously affected pregnancies have a risk of 50% of fetal CHB in subsequent pregnancies [31][3].
For anti-Ro antibody titers higher than 50 UI/mL, the risk of developing CHB is 5%, with a high risk of developing a complete atrioventricular (AV) block in the fetus [33][4]. It is important to know that asymptomatic mothers with higher titers of anti-Ro antibodies (without symptoms or signs of SLE and/or SS) can give birth to children with neonatal lupus. However, studies show that about one-half of these women will later develop an autoimmune disease, especially SS [34][5].
Cutaneous manifestations of neonatal lupus seem to appear in 7–16% of the newborns with anti-Ro and anti-La positive mothers and can be associated with the presence of anti-ribonucleoprotein (RNP) antibodies [35][6].
2.2. Molecular Mechanisms Leading to Fetal Complications
The precise molecular mechanism through which the anti-Ro/SSA and anti-La/SSB antibodies affect the fetal skin and heart is still an ongoing research topic. The presence of anti-Ro antibodies is necessary, but not sufficient to cause CHB. Current studies have shown that the development of neonatal lupus is influenced by the interaction between the transplacental passage of maternal antibodies, starting in the late first trimester or early second trimester and additive factors such as fetal genetic (specific human leukocyte antigen (HLA) alleles) and environmental factors. Such interactions could explain why these complications still remain rare, as the presence of even high titers of these antibodies alone is not enough to cause the disease (as previously mentioned, the maximum risk is 2% in nulliparous women) [36][7]. This theory is also supported by the discordance of heart block in identical twins, which suggests that in utero factors have also an important role to play in the pathogenesis of CHB [37][8].
Available immunohistochemistry data confirms that the permanent electrogenic disturbances are ultimately caused by fibrosis and calcification, but the ultimate pathway to fibrosis may be variable [38]. There are two theories regarding the molecular mechanism of the autoimmune induced CHB, the “apoptosis hypothesis” and the “Ca channel hypothesis” [39–41][9][10][11].
2.2.1. The “Apoptosis Hypothesis”
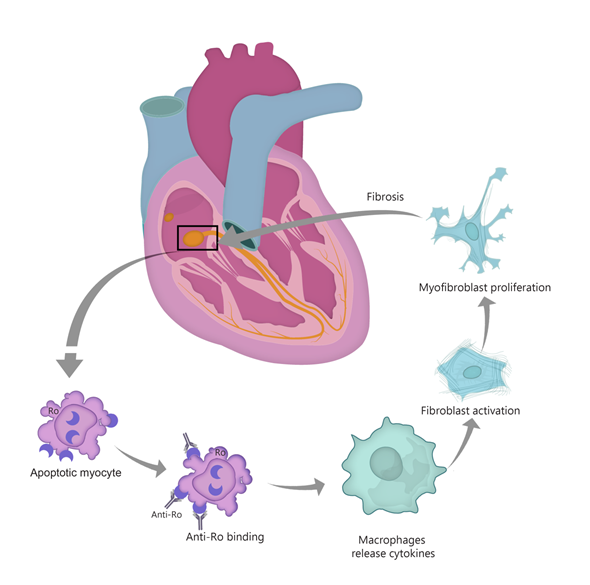
The first theory suggests that anti-Ro/SSA antibodies bind to surface antigens of cells undergoing apoptosis in the process of physiological remodeling (see Figure 1). Typically, Ro antigens are intracellular, but the process of apoptosis induces translocation of these antibodies to the surface of fetal cardiomyocytes. In normal conditions, the apoptotic cardiocytes are phagocytosed, but the immune complexes impair the clearance of these cells [42][12]. Apoptotic cell accumulation induces macrophage infiltration, activation and release of cytokines such as TNF-α and TGF-β [31,38,42–44][3][13][12][14][15]. TGF stimulates the differentiation of fibroblasts into myofibroblasts, leading to scar formation [38][13]. Thus, the fetal cardiac conduction tissue is exposed to an autoimmune reaction that causes inflammation and fibrosis, leading to irreversible CHB [39][9]. Interestingly, in children with autoimmune CHB, a certain polymorphism of the TGF gene was described, linked to increased fibrosis [45][16]. Moreover, data from in vitro studies show that anti-Ro–associated single-stranded RNA binds to macrophage Toll-like receptors (TLR) and thus triggers the aforementioned inflammatory and fibrosis cascade [46]. An important role seems to be played in this context by interferon (IFN), with highly expressed type I IFN response genes [46,47][17][18].
Figure 1. “Apoptosis hypothesis”. TGF-β—transforming growth factor β.
2.2.2. The “Calcium Channel Hypothesis”
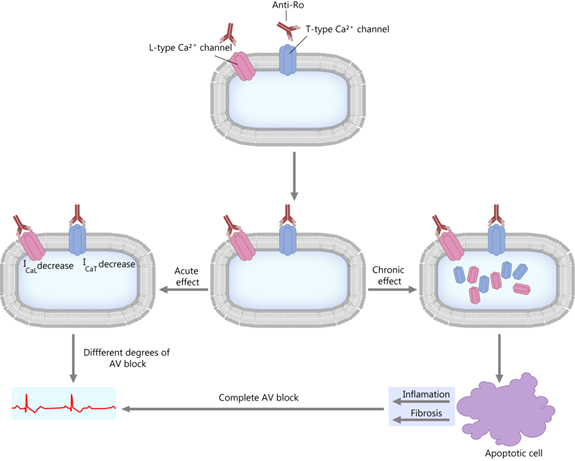
This theory is centered on the fact that the anti-Ro antibodies inhibit the calcium channels by direct interaction [39,48–50][9][19][20][21]. The anti-Ro/SSA antibodies can inhibit both the L-type and T-type calcium channels [39,51][9][22], which are essential to impulse propagation and conduction in the sinoatrial and AV nodes [43,51–53][14][22][23][24]. As a consequence, a short-term effect is a decrease in calcium currents, and a long-term effect is the internalization of the calcium channels (see Figure 2). These events lead to a disruption of the intracellular calcium handling, possibly contributing to the aforementioned apoptotic process and subsequent inflammation and fibrosis [39,50,54][9][21][25].
Figure 2. “Calcium hypothesis”. The short-term and long-term effects. AV block—atrioventricular block; ICaL; ICaT—calcium current through L-type and T-type Ca channels.
2.3. Is There a Relationship between the Titers of Anti-Ro/SSA and/or Anti-La/SSB Antibodies and the Incidence of Congenital Heart Block?
As previously discussed, experimental data show that the development of CHB is more frequent in newborns of women with high titers of anti-Ro/SSA and anti-La/SSB antibodies. In a prospective study of 186 antibody-exposed fetuses and newborns, Jaeggi E. et al. observed that the presence of maternal anti-Ro antibodies is responsible for the fetal tissue injury in a dose-dependent manner. The cutoff value was 50 U/mL, as measured by ELISA assay. As a result, many experts are recommending serial echocardiography only in women with high anti-Ro titers [33][4].
Another study conducted by Kan N. et al. including 232 pregnancies of anti-Ro antibodies positive mothers, proved that limiting serial fetal echocardiograms to women with high anti-Ro antibody levels is safe and cost-effective [55][26].
2.4. Clinical Manifestations of Neonatal Lupus
The two major clinical manifestations of neonatal lupus can be divided into cutaneous (typical rash) and cardiac findings.
2.4.1. Cutaneous Manifestations
The rash is frequently observed within a few weeks after birth (sometimes up to four months), but it can also be noticed at birth, which proves that its photosensitivity is not a mandatory characteristic [56][27]. Neiman A.R. et al. concluded that the rash is usually diagnosed around six weeks and it is self-limiting. It almost always disappears by six to eight months, lasting for an average of 17 weeks [57][28].
Moreover, the exposure to ultraviolet light is thought to induce or exacerbate the rash. It usually manifests with annular lesions or arcuate macules that are mainly located on the scalp and in the periorbital area, but also on other parts of the body such as the palms, the soles and the diaper area [58][29].
Another rare cutaneous manifestation is telangiectasia on the face or genitals. It can be observed in about 10% of patients and can occur between 6 to 12 months of age, not only as the first manifestation of the disease, but also localized in skin areas that were previously affected by the typical rash [59][30].
It is noteworthy that patients with cutaneous neonatal lupus are at increased risk of developing autoimmune diseases throughout their lives and this is the reason why some authors are recommending a continuous follow-up, especially prior to adolescence [60][31].
2.4.2. Cardiac Manifestations
The risk of developing first-, second- or third-degree AV block is one of the most threatening complications of the presence of anti-Ro/SSA antibodies. It commonly occurs between 18 to 24 weeks of gestation, which explains why mothers at risk should undergo more intensive fetal surveillance in this delicate period.
Isolated cases have been reported as early as 16 weeks, although another systematic review showed that 75% of cases were diagnosed during weeks 20 to 29 [31][3].
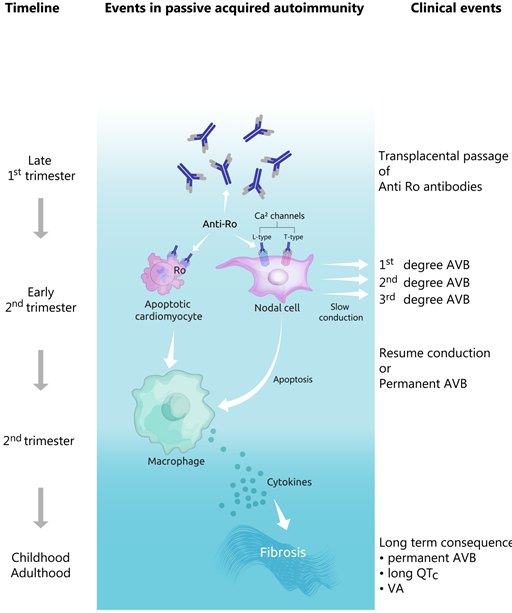
The first-degree AV block (defined by a PR interval which is longer than the upper limits of normal age) is considered to be a benign ECG finding, which appears in up to 6% of normal neonates and doesn’t lead to fetal bradycardia [61][32]. However, further fetal surveillance is recommended, as the first, as well as the second-degree AV blocks, may progress to more advanced blocks (see Figure 3). In addition, the autoimmune reaction affects the calcium channels present not only in the AV node, but also in the sinoatrial node. In this case, sinus node bradycardia may ensue.
Figure 3. Evolution of pathophysiological and clinical events in the development of congenital heart block. AVB—atrioventricular block, VA—ventricular arrhythmias.
The second-degree AV block may lead to fetal bradycardia, especially in advanced heart blocks in which two consecutive P waves fail to conduct to the ventricle, suggesting the presence of an important conduction block below the level of the AV node [62][33].
The third degree (or complete) AV block is the most serious and dramatic manifestation of neonatal lupus which leads to complete dissociation of the atrial and ventricular activity, as a result of the damage in the AV conduction pathways. In patients with third-degree AV block the atrial frequency is in the normal range, but the ventricular rate can vary between 50 and 80 beats/minute, as the rhythm is junctional or ventricular.
The resulting fetal bradycardia increases the risk of heart failure and sudden cardiac death. It is also responsible for the poor systemic perfusion, which can lead to hydrops fetalis, as a result of impaired ventricular filling and/or cardiac output [33][34][62,63]. Hydrops fetalis is defined as a collection of fluid in two body cavities (pleural, pericardial, ascites) or one body cavity plus anasarca [63,64][34][35].
The autoantibody-mediated heart blocks are usually associated with a structurally normal heart, although valvular lesions have been reported by some authors. For example, Cuneo BF et al. described three fetuses with ”unique” myocardial and conduction system diseases such as mitral and tricuspid valve chordal avulsion, sinoatrial and infrahisian conduction system disease or the association between sinus node dysfunction and atrial flutter [65][36]. When valvular pathology occurs, it is localized at the mitral or tricuspid valve, with regurgitation developing both pre- and postnatally and calls for urgent valve surgery [66][37].
There are also some other rare, but important cardiac manifestations of the presence of anti-Ro/SSA antibodies, such as sinus node dysfunction, atrial flutter, long QT interval, ventricular and junctional tachycardia and dilated cardiomyopathy [31,67][3][38]. However, the causality between these pathologies and the presence of maternal anti- Ro/SSA and anti- La/SSB antibodies is not firmly recognized.
Sinus bradycardia for example appeared in 3 of 76 fetuses (3.8%), for whom atrial rates were recorded by echocardiogram in a series of 187 fetuses with congenital heart block. Some authors consider that this disturbance is not permanent and that it could have a good prognosis [68,69][39][40]. On the other hand, persistent fetal sinus bradycardia may lead to serious cardiac complications when associated with endocardial fibroelastosis (EFE), ventricular dysfunction or AV block [69][40].
Dilated cardiomyopathy (DCM) occurs rarely, but has a high mortality rate [26,70][41][42]. It is characterized by an enlarged and poor-functioning left ventricle. There are two types of DCM (neonatal and late-onset). Neonatal DCM is associated with pericardial effusion, hydrops fetalis and endocardial fibroelastosis. Late-onset DCM is related to pacemaker implantation, in utero valve disease and non-European origin. [67][38]. Moreover, the risk factors for neonatal DCM do not predict the development of the late-onset form, which makes the long-term cardiovascular follow-up of these patients crucial for survival.
Endocardial fibroelastosis (EFE) is a condition that is characterized by diffuse thickening of the left ventricular endocardium secondary to the proliferation of fibrous and elastic tissue [71][43]. Nield LE et al. reported that 7 out of 13 children diagnosed with congenital heart block had EFE at presentation, while 6 developed EFE weeks to as long as five years after the diagnosis of the congenital heart block. Furthermore, 9 patients died and two underwent cardiac transplantation because of the presence of EFE [71][43]. The presence of EFE is associated with 51% mortality rate if it is concomitant with dilated cardiomyopathy, the mortality reaches 100% [26][41]. The diagnosis can be made in utero, by documenting areas of endocardial patchy echogenicity on the echocardiography examination.
2.4.3. Other Transient and more Rare Manifestations
Hematologic manifestations such as anemia, neutropenia, thrombocytopenia and aplastic anemia have been described in children with congenital heart block. However, hematological involvement seems to be almost always asymptomatic [56][27].
Hepatosplenomegaly, asymptomatic elevated liver enzymes and cholestasis are the main hepatic manifestations observed not only in fetuses with complete heart block, but also in those with cutaneous involvement [72][44].
Neurologic manifestations (hydrocephalus, nonspecific white matter changes and calcification of the basal ganglia) have been also reported, but their association with anti-Ro/SSA antibodies has not yet been proven.
2.5. Manifestations That Suggest the Diagnosis of Neonatal Lupus
The diagnosis of neonatal lupus requires the presence of both of the following elements: (1) the mother has positive anti-Ro/SSA, anti-La/SSB or anti-RNP antibodies and (2) the fetus or newborn develops heart block/typical rash/hepatic or hematologic manifestations in the absence of another etiology [73][45].
For these elements to be recognized, some recommendations regarding pre- and postnatal screening of children with CHB have been formulated [74][46]. The prenatal evaluation focuses not only on the maternal screening for anti-Ro/SSA and anti-La/SSB antibodies, but also on in utero surveillance for heart block.
It is strongly recommended that not only symptomatic, but also asymptomatic mothers carrying positive titers of anti-Ro/La antibodies should be more intensively monitored during pregnancy with frequent echocardiographic surveillance, especially during the period from 18 to 24 weeks of gestation.
On the other hand, the American Heart Association recommends a few additional weeks of serial examinations beginning from 16 weeks until 28 weeks of gestation [75][47]. The most efficient modality for screening and diagnostic evaluation of this bradyarrhythmia is the pulsed-Doppler fetal echocardiography. This non-invasive exploration should be performed weekly, as it has been observed that normal sinus rhythm can progress to complete block in seven days (during the high-risk period between 18 and 24 weeks of gestation). This investigation is of great importance in measuring the mechanical PR interval from the onset of the atrial contraction to ventricular contraction. Furthermore, echocardiography is of great importance in diagnosing not only first-degree heart block, but also endocardial fibroelastosis and valvular lesions [76][48].
Glickstein et al. proposed a validated reproducible method to measure the fetal mechanical PR interval, the equivalent to the electric PR interval on surface electrocardiography, by echocardiography with pulsed Doppler [76,77][48][49].
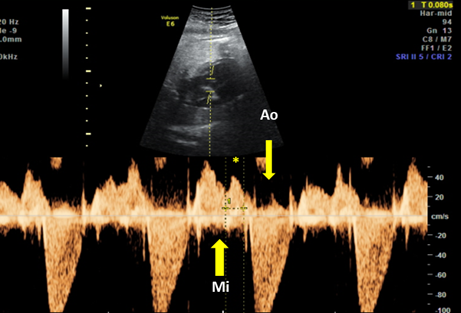
The fetal mechanical PR interval can be obtained during ultrasound examination of the fetal heart. It is recorded with the help of pulsed wave (PW) Doppler– the two-dimensional PW gate is set to 3–4 mm depending on the gestational age and placed distal to the mitral valve as such to include the origin of the left ventricle outflow tract at an angle of around 20°. With this approach, we will record spectral Doppler wave forms for both blood flows, in the mitral valve and aortic origin during a full cardiac cycle. The speed of image acquisition should be slowed (to 4–5 cm/s) in order to have a good representation of each waveform. During a normal cardiac cycle, the passive filling of the atria during generalized diastole (E wave), the active filling of ventricles during atrial systole (A wave) and blood ejection in the root of the aorta during ventricular systole will be documented (see Figure 4). The PR interval is the interval measured between the onset of atrial systole (mitral valve A‐wave) and the beginning of the aortic valve flow. Normal ranges during pregnancy depend on the gestational age, fetal heart rate and sex of the baby. These correlations have been studied prospectively by A. Wojakowski et al. [78][50]. A mean of 122.4 ms ± SD 10.3 ms is considered normal.
Figure 4. Pulsed wave Doppler trace used for mechanical PR interval measurement. Mi—transmitral flow (thick yellow arrow); Ao—transaortic flow (thin yellow arrow); (*)—normal PR interval—120 msec (between thin dotted yellow lines). Courtesy of Anca Panaitescu, Filantropia Clinical Hospital, Bucharest.
In a prospective trial of dexamethasone to prevent CHB progression in women with anti-Ro by Friedman et al. the definition of “abnormal” fetal Doppler mechanical PR interval was set a priori at three SD above the normal mean, to 150 ms [79][51]. However, none of the conduction measurements could predict the occurrence of CHB.
The two-dimensional ultrasound can be used in diagnosing specific arrhythmias, in evaluating cardiac anatomy and function, as well as in searching for signs of hydrops fetalis. Arrhythmias can be further characterized by using the M-mode ultrasonography, which detects atrial and ventricular wall motion and the relative timing of cardiac events (see clinical case [80][52]. Fetal bradycardia, the result of both second- and third- heart block, can be easily detected by routine fetal auscultation.
The weekly pulsed-Doppler fetal echocardiography is the preferred method of in utero surveillance for heart block. However, if not available, there is an alternative that consists of home monitoring for fetal bradycardia, twice per day, with a handheld Doppler system [80][52]. The postnatal testing is recommended for mothers of neonates with heart block in the absence of causal structural abnormalities, as most cases of congenital heart blocks appear as a result of the presence of anti-Ro/SSA and/or anti-La/SSB antibodies. In addition, children up to eight months of age presenting rash and/or any degree of heart block should also be tested for the aforementioned antibodies [36,81][7][53].
- Deutscher, S.L.; Harley, J.B.; Keene, J.D. Molecular analysis of the 60-kDa human Ro ribonucleoprotein. Natl. Acad. Sci. USA 1988, 85, 9479–9483.
- Xue, D.; Shi, H.; Smith, J.D.; Chen, X.; Noe, D.A.; Cedervall, T.; Yang, D.D.; Eynon, E.; Brash, D.E.; Kashgarian, M.; et al. A lupus-like syndrome develops in mice lacking the Ro 60-kDa protein, a major lupus autoantigen. Natl. Acad. Sci. USA 2003, 100, 7503–7508.
- Chan, E.K.; Hamel, J.C.; Buyon, J.P.; Tan, E.M. Molecular definition and sequence motifs of the 52-kD component of human SS-A/Ro autoantigen. Clin. Invest. 1991, 87, 68–76.
- Higgs, R.; Lazzari, E.; Wynne, C.; Gabhann, J.N.; Espinosa, A.; Wahren-Herlenius, M.; Jefferies, C.A. Self protection from anti-viral responses—Ro52 promotes degradation of the transcription factor IRF7 downstream of the viral toll-like receptors. PLoS ONE 2010, 5, e11776.
- Higgs, R.; Gabhann, J.N.; Larbi, N.B.; Breen, E.P.; Fitzgerald, K.A.; Jefferies, C.A. The E3 ubiquitin ligase Ro52 negatively regulates IFN-β production post-pathogen recognition by polyubiquitin-mediated degradation of IRF3. Immunol. 2008, 181, 1780–1786.
- Wada, K.; Niida, M.; Tanaka, M.; Kamitani, T. Ro52-mediated monoubiquitination of IKKβ down-regulates NF-κB signalling. Biochem. 2009, 146, 821–832.
- Bolland, S.; Garcia-Sastre, A. Vicious circle: Systemic autoreactivity in Ro52/TRIM21-deficient mice. Exp. Med. 2009, 206, 1647–1651.
- Eftekhari, P.; Sallé, L.; Lezoualc'h, F.; Mialet, J.; Gastineau, M.; Briand, J.P.; Isenberg, D.A.; Fournié, G.J.; Argibay, J.; Fischmeister, R.; et al. Anti-SSA/Ro52 autoantibodies blocking the cardiac 5-HT4 serotoninergic receptor could explain neonatal lupus congenital heart block. J. Immunol. 2000, 30, 2782–2790.
- Fok, V.; Friend, K.; Steitz, J.A. Epstein-Barr virus noncoding RNAs are confined to the nucleus, whereas their partner, the human La protein, undergoes nucleocytoplasmic shuttling. Cell Biol. 2006, 173, 319–325.
- Liu, Y.; Tan, H.; Tian, H.; Liang, C.; Chen, S.; Liu, Q. Autoantigen la promotes efficient RNAi, antiviral response, and transposon silencing by facilitating multiple-turnover RISC catalysis. Cell 2011, 44, 502–508.
- Solomon, D.H.; Kavanaugh, A.J.; Schur, P.H. Evidence-based guidelines for the use of immunologic tests: Antinuclear antibody testing,” Arthritis Rheum. 2002, 47, 434–444.
- Agmon-Levin, N.; Damoiseaux, J.; Kallenberg, C.; Sack, U.; Witte, T.; Herold, M.; Bossuyt, X.; Musset, L.; Cervera, R.; Plaza-Lopez, A.; et al. International recommendations for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. Rheum. Dis. 2014, 73, 17–23.
- Lazzaroni, M.G.; Dall’Ara, F.; Fredi, M.; Nalli, C.; Reggia, R.; Lojacono, A.; Ramazzotto, F.; Zatti, S.; Andreoli, L.; Tincani, A. A comprehensive review of the clinical approach to pregnancy and systemic lupus erythematosus. Autoimmun. 2016, 74, 106–117.
- Kyriakidis, N.C.; Kapsogeorgou, E.K.; Tzioufas, A.G. A comprehensive review of autoantibodies in primary Sjögren’s syndrome: Clinical phenotypes and regulatory mechanisms. Autoimmun. 2014, 51, 67–74.
- Retamozo, S.; Akasbi, M.; Brito-Zerón, P.; Bosch, X.; Bove, A.; Perez-de-Lis, M.; Jimenez, I.; Soto-Cardenas, M.J.; Gandía, M.; Diaz-Lagares, C.; et al. Anti-Ro52 antibody testing influences the classification and clinical characterisation of primary Sjögren’s syndrome. Exp. Rheumatol. 2012, 30, 686–692.
- Hudson, M.; Pope, J.; Mahler, M.; Tatibouet, S.; Steele, R.; Baron, M.; Fritzler, M.J.; Canadian Scleroderma Research Group. Clinical significance of antibodies to Ro52/TRIM21 in systemic sclerosis. Arthritis Res. Ther. 2012, 14, R50.
- Gunnarsson, R.; El-Hage, F.; Aaløkken, T.M.; Reiseter, S.; Lund, M.B.; Garen, T.; Norwegian MCTD Study Group, Molberg, Ø. Associations between anti-Ro52 antibodies and lung fibrosis in mixed connective tissue disease. Rheumatology 2016, 55, 103–108.
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol. 2017, 69, 35–45.
- Guo, Y.P.; Wang, C.G.; Liu, X.; Huang, Y.Q.; Guo, D.L.; Jing, X.Z.; Yuan, C.G.; Yang, S.; Liu, J.M.; Han, M.S.; et al. The prevalence of antinuclear antibodies in the general population of china: A cross-sectional study. Ther. Res. Clin. Exp. 2014, 76, 116–119.
- Satoh, M.; Chan, E.K.; Ho, L.A.; Rose, K.M.; Parks, C.G.; Cohn, R.D.; Jusko, T.A.; Walker, N.J.; Germolec, D.R.; Whitt, I.Z.; et al. Prevalence and sociodemographic correlates of antinuclear antibodies in the United States. Arthritis Rheum. 2012, 64, 2319–2327.
- Hayashi, N.; Koshiba, M.; Nishimura, K.; Sugiyama, D.; Nakamura, T.; Morinobu, S.; Kawano, S.; Kumagai, S. Prevalence of disease-specific antinuclear antibodies in general population: Estimates from annual physical examinations of residents of a small town over a 5-year period. Rheumatol. 2008, 18, 153–160.
- Arbuckle, M.R.; McClain, M.T.; Rubertone, M.V.; Scofield, R.H.; Dennis, G.J.; James, J.A.; Harley, J.B. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. Engl. J. Med. 2003, 349, 1526–1533.
- Theander, E.; Jonsson, R.; Sjöström, B.; Brokstad, K.; Olsson, P.; Henriksson, G. Prediction of Sjögren’s syndrome years before diagnosis and identification of patients with early onset and severe disease course by autoantibody profiling,” Arthritis Rheumatol. 2015, 67, 2427–2436.
- Sammaritano, L.R.; Bermas, B.L.; Chakravarty, E.E.; Chambers, C.; Clowse, M.E.; Lockshin, M.D.; Marder, W.; Guyatt, G.; Branch, D.W.; Buyon, J.; et al. 2020 American College of Rheumatology Guideline for the Management of Reproductive Health in Rheumatic and Musculoskeletal Diseases. Arthritis Care Res. 2020, 72, 461–488.
- Wainwright, B.; Bhan, R.; Trad, C.; Cohen, R.; Saxena, A.; Buyon, J.; Izmirly, P. Autoimmune-mediated congenital heart block. In Best Practice and Research: Clinical Obstetrics and Gynaecology; Bailliere Tindall Ltd.: London, UK, 2019.
- Izmirly, P.M.; Saxena, A.; Kim, M.Y.; Wang, D.; Sahl, S.K.; Llanos, C.; Friedman, D.; Buyon, J.P. Maternal and fetal factors associated with mortality and morbidity in a multi-racial/ethnic registry of anti-SSA/Ro-associated cardiac neonatal lupus. Circulation 2011, 124, 1927–1935.
- Lopes, L.M.; Tavares, G.M.; Damiano, A.P.; Lopes, M.A.; Aiello, V.D.; Schultz, R.; Zugaib, M. Perinatal outcome of fetal atrioventricular block one-hundred-sixteen cases from a single institution. Circulation 2008, 118, 1268–1275.
- Eronen, M.; Sirèn, M.K.; Ekblad, H.; Tikanoja, T.; Julkunen, H.; Paavilainen, T. Short- and long-term outcome of children with congenital complete heart block diagnosed in utero or as a newborn. Pediatrics 2000, 106, 86–91.
- Eliasson, H.; Sonesson, S.E.; Sharland, G.; Granath, F.; Simpson, J.M.; Carvalho, J.S.; Jicinska, H.; Tomek, V.; Dangel, J.; Zielinsky, P.; et al. Isolated atrioventricular block in the fetus: A retrospective, multinational, multicenter study of 175 patients. Circulation 2011, 124, 1919–1926.
- Ho, A.; Gordon, P.; Rosenthal, E.; Simpson, J.; Miller, O.; Sharland, G. Isolated complete heart block in the fetus. J. Cardiol. 2015, 116, 142–147.
- Brito-Zerón, P.; Izmirly, P.M.; Ramos-Casals, M.; Buyon, J.P.; Khamashta, M.A. The clinical spectrum of autoimmune congenital heart block. Rev. Rheumatol. 2015, 11, 301–312.
- Panaitescu, A.M.; Nicolaides, K. Maternal autoimmune disorders and fetal defects. In Journal of Maternal-Fetal and Neonatal Medicine; Taylor and Francis Ltd.: London, UK, 2018; Volume 31, pp. 1798–1806.
- Jaeggi, E.; Laskin, C.; Hamilton, R.; Silverman, E. The importance of the level of maternal anti-Ro/SSA antibodies as a prognostic marker of the development of cardiac neonatal lupus erythematosus: A prospective study of 186 antibody-exposed fetuses and infants. Am. Coll. Cardiol. 2010, 55, 2778–2784.
- Rivera, T.L.; Izmirly, P.M.; Birnbaum, B.K.; Byrne, P.; Brauth, J.B.; Katholi, M.; Kim, M.Y.; Fischer, J.; Clancy, R.M.; Buyon, J.P. Disease progression in mothers of children enrolled in the Research Registry for Neonatal Lupus. Rheum. Dis. 2009, 68, 828–835.
- Provost, T.T.; Watson, R.; Gammon, W.R.; Radowsky, M.; Harley, J.B.; Reichlin, M. The neonatal lupus syndrome associated with U1RNP (nRNP) antibodies. Engl. J. Med. 1987, 316, 1135–1138.
- Buyon, J.P.; Clancy, R.M.; Friedman, D.M. Cardiac manifestations of neonatal lupus erythematosus: Guidelines to management, integrating clues from the bench and bedside. Clin. Pract. Rheumatol. 2009, 5, 139–148.
- Cooley, H.M.; Keech, C.L.; Melny, B.J.; Menahem, S.; Morahan, G.; Kay, T.W. Monozygotic twins discordant for congenital complete heart block. Arthritis Rheum. 1997, 40, 381–384.
- Clancy, R.M.; Kapur, R.P.; Molad, Y.; Askanase, A.D.; Buyon, J.P. Immunohistologic evidence supports apoptosis, lgG deposition, and novel macrophage/fibroblast crosstalk in the pathologic cascade leading to congenital heart block. Arthritis Rheum. 2004, 50, 173–182.
- Qu, Y.S.; Lazzerini, P.E.; Capecchi, P.L.; Laghi-Pasini, F.; El Sherif, N.; Boutjdir, M. Autoimmune calcium channelopathies and cardiac electrical abnormalities. Cardiovasc. Med. 2019, 6, 54.
- Izmirly, P.; Saxena, A.; Buyon, J.P. Progress in the pathogenesis and treatment of cardiac manifestations of neonatal lupus. Opin. Rheumatol. 2017, 29, 467–472, Lippincott Williams and Wilkins: Philadelphia, PA, USA.
- Boutjdir, M. Molecular and ionic basis of congenital complete heart block. Trends Cardiovasc. Med. 2000, 10, 114–122.
- Clancy, R.M.; Neufing, P.J.; Zheng, P.; O’Mahony, M.; Nimmerjahn, F.; Gordon, T.P.; Buyon, J.P. Impaired clearance of apoptotic cardiocytes is linked to anti-SSA/Ro and -SSB/La antibodies in the pathogenesis of congenital heart block. Clin. Invest. 2006, 116, 2413–2422.
- Miranda-Carús, M.E.; Askanase, A.D.; Clancy, R.M.; Di Donato, F.; Chou, T.M.; Libera, M.R.; Chan, E.K.; Buyon, J.P. Anti-SSA/Ro and Anti-SSB/La Autoantibodies Bind the Surface of Apoptotic Fetal Cardiocytes and Promote Secretion of TNF-α by Macrophages. Immunol. 2000, 165, 5345–5351.
- Buyon, J.P.; Clancy, R.M. Autoantibody-associated congenital heart block: TGFh and the road to scar. Rev. 2004, 4, 1–7.
- Clancy, R.M.; Backer, C.B.; Yin, X.; Kapur, R.P.; Molad, Y.; Buyon, J.P. Cytokine polymorphisms and histologic expression in autopsy studies: Contribution of TNF-α and TGF-β1 to the pathogenesis of autoimmune-associated congenital heart block. Immunol. 2003, 171, 3253–3261.
- Clancy, R.M.; Halushka, M.; Rasmussen, S.E.; Lhakhang, T.; Chang, M.; Buyon, J.P. Siglec-1 macrophages and the contribution of IFN to the development of autoimmune congenital heart block. Immunol. 2019, 202, 48–55.
- Hedlund, M.; Thorlacius, G.E.; Ivanchenko, M.; Ottosson, V.; Kyriakidis, N.; Lagnefeldt, L.; Tingström, J.; Sirsjö, A.; Bengtsson, A.A.; Aronsson, E.; et al. Type i IFN system activation in newborns exposed to Ro/SSA and La/SSB autoantibodies in utero. RMD Open 2020, 6, DOI: 10.1136/rmdopen-2019-000989.
- Boutjdir, M.; Chen, L.; Zhang, Z.H.; Tseng, C.E.; El-Sherif, N.; Buyon, J.P. Serum and immunoglobulin G from the mother of a child with congenital heart block induce conduction abnormalities and inhibit L-type calcium channels in a rat heart model. Res. 1998, 44, 11–19.
- Boutjdir, M.; Chen, L.; Zhang, Z.H.; Tseng, C.E.; DiDonato, F.; Rashbaum, W.; Morris, A.; El-Sherif, N.; Buyon, J.P. Arrhythmogenicity of lgG and anti-52-kD SSA/Ro affinity-purified antibodies from mothers of children with congenital heart block. Res. 1997, 80, 354–362.
- Karnabi, E.; Qu, Y.; Wadgaonkar, R.; Mancarella, S.; Yue, Y.; Chahine, M.; Clancy, R.M.; Buyon, J.P.; Boutjdir, M. Congenital heart block: Identification of autoantibody binding site on the extracellular loop (domain I, S5-S6) of α1D L-type Ca channel. Autoimmun. 2010, 34, 80–86.
- Lazzerini, P.E.; Capecchi, P.L.; Laghi-Pasini, F.; Boutjdir, M. Autoimmune channelopathies as a novel mechanism in cardiac arrhythmias. Rev. Cardiol. 2017, 14, 521–535.
- Jobling, K.; Rajabally, H.; Ng, W.F. Anti-Ro antibodies and complete heart block in adults with Sjögren’s syndrome. J. Rheumatol. 2018, 5, 194–196.
- Xiao, G.Q.; Hu, K.; Boutjdir, M. Direct inhibition of expressed cardiac L- and T-type calcium channels by IgG from mothers whose children have congenital heart block. Circulation 2001, 103, 1599–1604.
- Ambrosi, A.; Wahren-Herlenius, M. Congenital heart block: Evidence for a pathogenic role of maternal autoantibodies. Arthritis Res. Ther. 2012, 14, 208.
- Kan, N.; Silverman, E.D.; Kingdom, J.; Dutil, N.; Laskin, C.; Jaeggi, E. Serial echocardiography for immune-mediated heart disease in the fetus: Results of a risk-based prospective surveillance strategy. Diagn. 2017, 37, 375–382.
- Silverman, E.; Jaeggi, E. Non-cardiac manifestations of neonatal lupus erythematosus. In Scandinavian Journal of Immunology; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2010; Volume 72, pp. 223–225.
- Neiman, A.R.; Lee, L.A.; Weston, W.L.; Buyon, J.P. Cutaneous manifestations of neonatal lupus without heart block: Characteristics of mothers and children enrolled in a national registry. Pediatr. 2000, 137, 674–680.
- Chadha, A.; Jahnke, M. Common neonatal rashes. Ann. 2019, 48, e16–e22.
- Guinovart, R.M.; Vicente, A.; Rovira, C.; Suñol, M.; González-Enseñat, M.A. Facial telangiectasia: An unusual manifestation of neonatal lupus erythematosus. Lupus 2012, 21, 552–555.
- Martin, V.; Lee, L.A.; Askanase, A.D.; Katholi, M.; Buyon, J.P. Long-term followup of children with neonatal lupus and their unaffected siblings. Arthritis Rheum. 2002, 46, 2377–2383.
- Roberts, N.K.; Gelband, H. Arrhythmias in the neonate. In Cardiac Arrhythmias in the Neonate, Infant, and Child; Appleton-Century-Crofts: New York, NY, USA, 1977; p. 533.
- Baruteau, A.E.; Pass, R.H.; Thambo, J.B.; Behaghel, A.; Le Pennec, S.; Perdreau, E.; Combes, N.; Liberman, L.; McLeod, C.J. Congenital and childhood atrioventricular blocks: Pathophysiology and contemporary management. J. Pediatr. 2016, 175, 1235–1248, Springer: Berlin, Germany.
- Sekar, R. Hydrops Fetalis. In Complications of Pregnancy; IntechOpen: London, UK, 2019.
- Derderian, S.C.; Jeanty, C.; Fleck, S.R.; Cheng, L.S.; Peyvandi, S.; Moon-Grady, A.J.; Farrell, J.; Hirose, S.; Gonzalez, J.; Keller, R.L.; et al.The many faces of hydrops. Pediatr. Surg. 2015, 50, 50–54.
- Cuneo, B.F.; Strasburger, J.F.; Niksch, A.; Ovadia, M.; Wakai, R.T. An expanded phenotype of maternal SSASSB antibody-associated fetal cardiac disease. Matern. Neonatal Med. 2009, 22, 233–238.
- Cuneo, B.F.; Fruitman, D.; Benson, D.W.; Ngan, B.Y.; Liske, M.R.; Wahren-Herlineus, M.; Ho, S.Y.; Jaeggi, E. Spontaneous rupture of atrioventricular valve tensor apparatus as late manifestation of anti-Ro/SSA antibody-mediated cardiac disease. J. Cardiol. 2011, 107, 761–766.
- Morel, N.; Lévesque, K.; Maltret, A.; Baron, G.; Hamidou, M.; Orquevaux, P.; Piette, J.C.; Barriere, F.; Le Bidois, J.; Fermont, L. et al. Incidence, risk factors, and mortality of neonatal and late-onset dilated cardiomyopathy associated with cardiac neonatal lupus. J. Cardiol. 2017, 248, 263–269.
- Askanase, A.D.; Friedman, D.M.; Copel, J.; Dische, M.R.; Dubin, A.; Starc, T.J.; Katholi, M.C.; Buyon, J.P. Spectrum and progression of conduction abnormalities in infants born to mothers with anti-SSA/Ro-SSB/La antibodies. Lupus 2002, 11, 145–151.
- Chockalingam, P.; Jaeggi, E.T.; Rammeloo, L.A.; Haak, M.C.; Adama Van Scheltema, P.N.; Breur, J.M.P.J.; Bartelings, M.M.; Clur, S.A.B.; Blom, N.A. Persistent fetal sinus bradycardia associated with maternal anti-SSA/Ro and anti-SSB/La antibodies. Rheumatol. 2011, 38, 2682–2685.
- Moak, J.P.; Barron, K.S.; Hougen, T.J.; Wiles, H.B.; Balaji, S.; Sreeram, N.; Cohen, M.H.; Nordenberg, A.; Van Hare, G.F.; Friedman, R.A.; et al. Congenital heart block: Development of late-onset cardiomyopathy, a previously underappreciated sequela. Am. Coll. Cardiol. 2001, 37, 238–242.
- Nield, L.E.; Silverman, E.D.; Taylor, G.P., Smallhorn, J.F.; Mullen, J.B.; Silverman, N.H.; Finley, J.P.; Law, Y.M.; Human, D.G.; Seaward, P.G.; et al. Maternal anti-Ro and anti-La antibody—Associated endocardial fibroelastosis. Circulation 2002, 105, 843–848.
- Lee, L.A.; Sokol, R.J.; Buyon, J.P. Hepatobiliary disease in neonatal lupus: Prevalence and clinical characteristics in cases enrolled in a national registry. Pediatrics 2002, 109, e11.
- Lee, L.A. Neonatal Lupus: Clinical Features and Management. Drugs 2004, 6, 71–78.
- Olney, R.S.; Ailes, E.C.; Sontag, M.K. Detection of critical congenital heart defects: Review of contributions from prenatal and newborn screening. Perinatol. 2015, 39, 230–237.
- Donofrio, M.T.; Moon-Grady, A.J.; Hornberger, L.K.; Copel, J.A.; Sklansky, M.S.; Abuhamad, A.; Cuneo, B.F.; Huhta, J.C.; Jonas, R.A.; Krishnan, A.; et al. Diagnosis and treatment of fetal cardiac disease: A scientific statement from the american heart association. Circulation 2014, 129, 2183–2242.
- Glickstein, J.S.; Buyon, J.; Friedman, D. Pulsed Doppler echocardiographic assessment of the fetal PR interval. J. Cardiol. 2000, 86, 236–239.
- Glickstein, J.; Buyon, J.; Kim, M.; Friedman, D.; Abuhamad, A.; Branch, D.W.; Sullivan, A.; Cohen, M.; Copel, J.; Donofrio, M.; et al. The fetal Doppler mechanical PR interval: A validation study. Fetal Diagn. Ther. 2004, 19, 31–34.
- Wojakowski, A.; Izbizky, G.; Carcano, M.E.; Aiello, H.; Marantz, P.; Otaño, L. Fetal Doppler mechanical PR interval: Correlation with fetal heart rate, gestational age and fetal sex. Ultrasound Obstet. Gynecol. 2009, 34, 538–542.
- Friedman, D.M.; Kim, M.Y.; Copel, J.A.; Davis, C.; Phoon, C.K.L.; Glickstein, J.S.; Buyon, J.P. Utility of cardiac monitoring in fetuses at risk for congenital heart block: The PR interval and dexamethasone evaluation (PRIDE) prospective study. Circulation 2008, 117, 485–493.
- Pruetz, J.D.; Miller, J.C.; Loeb, G.E.; Silka, M.J.; Bar-Cohen, Y.; Chmait, R.H. Prenatal diagnosis and management of congenital complete heart block. Birth Defects Res. 2019, 111, 380–388.
- Jaeggi, E.T.; Hamilton, R.M.; Silverman, E.D.; Zamora, S.A.; Hornberger, L.K. Outcome of children with fetal, neonatal or childhood diagnosis of isolated congenital atrioventricular block. Am. Coll. Cardiol. 2002, 39, 130–137.
References
- Wainwright, B.; Bhan, R.; Trad, C.; Cohen, R.; Saxena, A.; Buyon, J.; Izmirly, P. Autoimmune-mediated congenital heart block. In Best Practice and Research: Clinical Obstetrics and Gynaecology; Bailliere Tindall Ltd.: London, UK, 2019.
- Panaitescu, A.M.; Nicolaides, K. Maternal autoimmune disorders and fetal defects. In Journal of Maternal-Fetal and Neonatal Medicine; Taylor and Francis Ltd.: London, UK, 2018; Volume 31, pp. 1798–1806.
- Brito-Zerón, P.; Izmirly, P.M.; Ramos-Casals, M.; Buyon, J.P.; Khamashta, M.A. The clinical spectrum of autoimmune congenital heart block. Rev. Rheumatol. 2015, 11, 301–312.
- Jaeggi, E.; Laskin, C.; Hamilton, R.; Silverman, E. The importance of the level of maternal anti-Ro/SSA antibodies as a prognostic marker of the development of cardiac neonatal lupus erythematosus: A prospective study of 186 antibody-exposed fetuses and infants. Am. Coll. Cardiol. 2010, 55, 2778–2784.
- Rivera, T.L.; Izmirly, P.M.; Birnbaum, B.K.; Byrne, P.; Brauth, J.B.; Katholi, M.; Kim, M.Y.; Fischer, J.; Clancy, R.M.; Buyon, J.P. Disease progression in mothers of children enrolled in the Research Registry for Neonatal Lupus. Rheum. Dis. 2009, 68, 828–835.
- Provost, T.T.; Watson, R.; Gammon, W.R.; Radowsky, M.; Harley, J.B.; Reichlin, M. The neonatal lupus syndrome associated with U1RNP (nRNP) antibodies. Engl. J. Med. 1987, 316, 1135–1138.
- Buyon, J.P.; Clancy, R.M.; Friedman, D.M. Cardiac manifestations of neonatal lupus erythematosus: Guidelines to management, integrating clues from the bench and bedside. Clin. Pract. Rheumatol. 2009, 5, 139–148.
- Cooley, H.M.; Keech, C.L.; Melny, B.J.; Menahem, S.; Morahan, G.; Kay, T.W. Monozygotic twins discordant for congenital complete heart block. Arthritis Rheum. 1997, 40, 381–384.
- Qu, Y.S.; Lazzerini, P.E.; Capecchi, P.L.; Laghi-Pasini, F.; El Sherif, N.; Boutjdir, M. Autoimmune calcium channelopathies and cardiac electrical abnormalities. Cardiovasc. Med. 2019, 6, 54.
- Izmirly, P.; Saxena, A.; Buyon, J.P. Progress in the pathogenesis and treatment of cardiac manifestations of neonatal lupus. Opin. Rheumatol. 2017, 29, 467–472, Lippincott Williams and Wilkins: Philadelphia, PA, USA.
- Boutjdir, M. Molecular and ionic basis of congenital complete heart block. Trends Cardiovasc. Med. 2000, 10, 114–122.
- Clancy, R.M.; Neufing, P.J.; Zheng, P.; O’Mahony, M.; Nimmerjahn, F.; Gordon, T.P.; Buyon, J.P. Impaired clearance of apoptotic cardiocytes is linked to anti-SSA/Ro and -SSB/La antibodies in the pathogenesis of congenital heart block. Clin. Invest. 2006, 116, 2413–2422.
- Clancy, R.M.; Kapur, R.P.; Molad, Y.; Askanase, A.D.; Buyon, J.P. Immunohistologic evidence supports apoptosis, lgG deposition, and novel macrophage/fibroblast crosstalk in the pathologic cascade leading to congenital heart block. Arthritis Rheum. 2004, 50, 173–182.
- Miranda-Carús, M.E.; Askanase, A.D.; Clancy, R.M.; Di Donato, F.; Chou, T.M.; Libera, M.R.; Chan, E.K.; Buyon, J.P. Anti-SSA/Ro and Anti-SSB/La Autoantibodies Bind the Surface of Apoptotic Fetal Cardiocytes and Promote Secretion of TNF-α by Macrophages. Immunol. 2000, 165, 5345–5351.
- Buyon, J.P.; Clancy, R.M. Autoantibody-associated congenital heart block: TGFh and the road to scar. Rev. 2004, 4, 1–7.
- Clancy, R.M.; Backer, C.B.; Yin, X.; Kapur, R.P.; Molad, Y.; Buyon, J.P. Cytokine polymorphisms and histologic expression in autopsy studies: Contribution of TNF-α and TGF-β1 to the pathogenesis of autoimmune-associated congenital heart block. Immunol. 2003, 171, 3253–3261.
- Clancy, R.M.; Halushka, M.; Rasmussen, S.E.; Lhakhang, T.; Chang, M.; Buyon, J.P. Siglec-1 macrophages and the contribution of IFN to the development of autoimmune congenital heart block. Immunol. 2019, 202, 48–55.
- Hedlund, M.; Thorlacius, G.E.; Ivanchenko, M.; Ottosson, V.; Kyriakidis, N.; Lagnefeldt, L.; Tingström, J.; Sirsjö, A.; Bengtsson, A.A.; Aronsson, E.; et al. Type i IFN system activation in newborns exposed to Ro/SSA and La/SSB autoantibodies in utero. RMD Open 2020, 6, DOI: 10.1136/rmdopen-2019-000989.
- Boutjdir, M.; Chen, L.; Zhang, Z.H.; Tseng, C.E.; El-Sherif, N.; Buyon, J.P. Serum and immunoglobulin G from the mother of a child with congenital heart block induce conduction abnormalities and inhibit L-type calcium channels in a rat heart model. Res. 1998, 44, 11–19.
- Boutjdir, M.; Chen, L.; Zhang, Z.H.; Tseng, C.E.; DiDonato, F.; Rashbaum, W.; Morris, A.; El-Sherif, N.; Buyon, J.P. Arrhythmogenicity of lgG and anti-52-kD SSA/Ro affinity-purified antibodies from mothers of children with congenital heart block. Res. 1997, 80, 354–362.
- Karnabi, E.; Qu, Y.; Wadgaonkar, R.; Mancarella, S.; Yue, Y.; Chahine, M.; Clancy, R.M.; Buyon, J.P.; Boutjdir, M. Congenital heart block: Identification of autoantibody binding site on the extracellular loop (domain I, S5-S6) of α1D L-type Ca channel. Autoimmun. 2010, 34, 80–86.
- Lazzerini, P.E.; Capecchi, P.L.; Laghi-Pasini, F.; Boutjdir, M. Autoimmune channelopathies as a novel mechanism in cardiac arrhythmias. Rev. Cardiol. 2017, 14, 521–535.
- Jobling, K.; Rajabally, H.; Ng, W.F. Anti-Ro antibodies and complete heart block in adults with Sjögren’s syndrome. J. Rheumatol. 2018, 5, 194–196.
- Xiao, G.Q.; Hu, K.; Boutjdir, M. Direct inhibition of expressed cardiac L- and T-type calcium channels by IgG from mothers whose children have congenital heart block. Circulation 2001, 103, 1599–1604.
- Ambrosi, A.; Wahren-Herlenius, M. Congenital heart block: Evidence for a pathogenic role of maternal autoantibodies. Arthritis Res. Ther. 2012, 14, 208.
- Kan, N.; Silverman, E.D.; Kingdom, J.; Dutil, N.; Laskin, C.; Jaeggi, E. Serial echocardiography for immune-mediated heart disease in the fetus: Results of a risk-based prospective surveillance strategy. Diagn. 2017, 37, 375–382.
- Silverman, E.; Jaeggi, E. Non-cardiac manifestations of neonatal lupus erythematosus. In Scandinavian Journal of Immunology; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2010; Volume 72, pp. 223–225.
- Neiman, A.R.; Lee, L.A.; Weston, W.L.; Buyon, J.P. Cutaneous manifestations of neonatal lupus without heart block: Characteristics of mothers and children enrolled in a national registry. Pediatr. 2000, 137, 674–680.
- Chadha, A.; Jahnke, M. Common neonatal rashes. Ann. 2019, 48, e16–e22.
- Guinovart, R.M.; Vicente, A.; Rovira, C.; Suñol, M.; González-Enseñat, M.A. Facial telangiectasia: An unusual manifestation of neonatal lupus erythematosus. Lupus 2012, 21, 552–555.
- Martin, V.; Lee, L.A.; Askanase, A.D.; Katholi, M.; Buyon, J.P. Long-term followup of children with neonatal lupus and their unaffected siblings. Arthritis Rheum. 2002, 46, 2377–2383.
- Roberts, N.K.; Gelband, H. Arrhythmias in the neonate. In Cardiac Arrhythmias in the Neonate, Infant, and Child; Appleton-Century-Crofts: New York, NY, USA, 1977; p. 533.
- Baruteau, A.E.; Pass, R.H.; Thambo, J.B.; Behaghel, A.; Le Pennec, S.; Perdreau, E.; Combes, N.; Liberman, L.; McLeod, C.J. Congenital and childhood atrioventricular blocks: Pathophysiology and contemporary management. J. Pediatr. 2016, 175, 1235–1248, Springer: Berlin, Germany.
- Sekar, R. Hydrops Fetalis. In Complications of Pregnancy; IntechOpen: London, UK, 2019.
- Derderian, S.C.; Jeanty, C.; Fleck, S.R.; Cheng, L.S.; Peyvandi, S.; Moon-Grady, A.J.; Farrell, J.; Hirose, S.; Gonzalez, J.; Keller, R.L.; et al.The many faces of hydrops. Pediatr. Surg. 2015, 50, 50–54.
- Cuneo, B.F.; Strasburger, J.F.; Niksch, A.; Ovadia, M.; Wakai, R.T. An expanded phenotype of maternal SSASSB antibody-associated fetal cardiac disease. Matern. Neonatal Med. 2009, 22, 233–238.
- Cuneo, B.F.; Fruitman, D.; Benson, D.W.; Ngan, B.Y.; Liske, M.R.; Wahren-Herlineus, M.; Ho, S.Y.; Jaeggi, E. Spontaneous rupture of atrioventricular valve tensor apparatus as late manifestation of anti-Ro/SSA antibody-mediated cardiac disease. J. Cardiol. 2011, 107, 761–766.
- Morel, N.; Lévesque, K.; Maltret, A.; Baron, G.; Hamidou, M.; Orquevaux, P.; Piette, J.C.; Barriere, F.; Le Bidois, J.; Fermont, L. et al. Incidence, risk factors, and mortality of neonatal and late-onset dilated cardiomyopathy associated with cardiac neonatal lupus. J. Cardiol. 2017, 248, 263–269.
- Askanase, A.D.; Friedman, D.M.; Copel, J.; Dische, M.R.; Dubin, A.; Starc, T.J.; Katholi, M.C.; Buyon, J.P. Spectrum and progression of conduction abnormalities in infants born to mothers with anti-SSA/Ro-SSB/La antibodies. Lupus 2002, 11, 145–151.
- Chockalingam, P.; Jaeggi, E.T.; Rammeloo, L.A.; Haak, M.C.; Adama Van Scheltema, P.N.; Breur, J.M.P.J.; Bartelings, M.M.; Clur, S.A.B.; Blom, N.A. Persistent fetal sinus bradycardia associated with maternal anti-SSA/Ro and anti-SSB/La antibodies. Rheumatol. 2011, 38, 2682–2685.
- Izmirly, P.M.; Saxena, A.; Kim, M.Y.; Wang, D.; Sahl, S.K.; Llanos, C.; Friedman, D.; Buyon, J.P. Maternal and fetal factors associated with mortality and morbidity in a multi-racial/ethnic registry of anti-SSA/Ro-associated cardiac neonatal lupus. Circulation 2011, 124, 1927–1935.
- Moak, J.P.; Barron, K.S.; Hougen, T.J.; Wiles, H.B.; Balaji, S.; Sreeram, N.; Cohen, M.H.; Nordenberg, A.; Van Hare, G.F.; Friedman, R.A.; et al. Congenital heart block: Development of late-onset cardiomyopathy, a previously underappreciated sequela. Am. Coll. Cardiol. 2001, 37, 238–242.
- Nield, L.E.; Silverman, E.D.; Taylor, G.P., Smallhorn, J.F.; Mullen, J.B.; Silverman, N.H.; Finley, J.P.; Law, Y.M.; Human, D.G.; Seaward, P.G.; et al. Maternal anti-Ro and anti-La antibody—Associated endocardial fibroelastosis. Circulation 2002, 105, 843–848.
- Lee, L.A.; Sokol, R.J.; Buyon, J.P. Hepatobiliary disease in neonatal lupus: Prevalence and clinical characteristics in cases enrolled in a national registry. Pediatrics 2002, 109, e11.
- Lee, L.A. Neonatal Lupus: Clinical Features and Management. Drugs 2004, 6, 71–78.
- Olney, R.S.; Ailes, E.C.; Sontag, M.K. Detection of critical congenital heart defects: Review of contributions from prenatal and newborn screening. Perinatol. 2015, 39, 230–237.
- Donofrio, M.T.; Moon-Grady, A.J.; Hornberger, L.K.; Copel, J.A.; Sklansky, M.S.; Abuhamad, A.; Cuneo, B.F.; Huhta, J.C.; Jonas, R.A.; Krishnan, A.; et al. Diagnosis and treatment of fetal cardiac disease: A scientific statement from the american heart association. Circulation 2014, 129, 2183–2242.
- Glickstein, J.S.; Buyon, J.; Friedman, D. Pulsed Doppler echocardiographic assessment of the fetal PR interval. J. Cardiol. 2000, 86, 236–239.
- Glickstein, J.; Buyon, J.; Kim, M.; Friedman, D.; Abuhamad, A.; Branch, D.W.; Sullivan, A.; Cohen, M.; Copel, J.; Donofrio, M.; et al. The fetal Doppler mechanical PR interval: A validation study. Fetal Diagn. Ther. 2004, 19, 31–34.
- Wojakowski, A.; Izbizky, G.; Carcano, M.E.; Aiello, H.; Marantz, P.; Otaño, L. Fetal Doppler mechanical PR interval: Correlation with fetal heart rate, gestational age and fetal sex. Ultrasound Obstet. Gynecol. 2009, 34, 538–542.
- Friedman, D.M.; Kim, M.Y.; Copel, J.A.; Davis, C.; Phoon, C.K.L.; Glickstein, J.S.; Buyon, J.P. Utility of cardiac monitoring in fetuses at risk for congenital heart block: The PR interval and dexamethasone evaluation (PRIDE) prospective study. Circulation 2008, 117, 485–493.
- Pruetz, J.D.; Miller, J.C.; Loeb, G.E.; Silka, M.J.; Bar-Cohen, Y.; Chmait, R.H. Prenatal diagnosis and management of congenital complete heart block. Birth Defects Res. 2019, 111, 380–388.
- Jaeggi, E.T.; Hamilton, R.M.; Silverman, E.D.; Zamora, S.A.; Hornberger, L.K. Outcome of children with fetal, neonatal or childhood diagnosis of isolated congenital atrioventricular block. Am. Coll. Cardiol. 2002, 39, 130–137.