The schemechanisms behind the reduction in muscle pyruvate dehydrogenase complex (PDC) controlled carbohydrate (CHO) oxidation during chronic high-fat dietary intake (HFDI) are poorly understood, as is the basis points to potentially important roles for PPAR delta and FOXO1 in the palmitate-induced increase in myotube PDK4 protein expression and linked reductions in cellular glucose uptake, PDC activity and flux, and rates of CHO derived oxidation restove ATP generation during muscle contraction. To get an insight into these mechanisms, C2C12 myotubes were treated with palmitate or without (control) for 16 hrs in the presence and absence of electrical pulse stimulation (EPS). Compared to control, palmitate reduced cell glucose uptake,. Activation of PPAR delta with natural (free fatty acids) or synthetic ligands have been associated with increases in muscle PDK4 mRNA and protein expression, which would thereby inhibit PDC activity, acetylcarnitine accumulation and glucose-derived mitochondrial ATP production and increased PDK4, PPARa and PPARd protein expression and reduced the whole-cell p-FOXO1/t-FOXO1 ratio. Conversely, chronic EPS rescued palmitate-induced inhibition of CHO oxidation, reflected by increased glucose uptake, PDC activity and glucose-derived mitochondrial ATP production compared to palmitate alone. EPS was also associated with less PDK4 and PPARd proteins, and lower nuclear p-FOXO1/t-FOXO1 ratio normalised to the cytoplasmic ratio, but with no changes in PPARa protein. Collectively, these data suggest that HFDI reduces the rate of CHO oxidation through inhibi. In the case of FOXO1, dephosphorylation (activation) results in its translocation from the cytosol to the nucleus, where it can bind directly to the promoter region of the PDK4 gene. Moreover, FOXO1 dephosphorylation appears to be coupled via a signalling cascade to changes in circulatory FFA and/or insulin availability, thereby tying muscle PDK4 gene transcription (amongst others) to substrate availability. Furthermore, the finding that 16 hrs of EPS calcium release rescued this palmitate-induced dysregulation of cellular CHO metabolism via modulation of PDC activity and its flux by PPARd and FOXO1 transcription factors meditated rise in PDK4the same molecular and cellular events, alludes to the mechanisms by which chronic physical activity will protein. Conversely, EPS rescued thesct skeletal muscle against lipid-induced muscle metabolic events by modulating the same transcription factorsinflexibility and IR.
- fuel selection
- PPAR delta
- FOXO1
- PDK4
- PDC activity
1. Introduction
Introduction
Obesity and chronically elevated levels of circulating free fatty acids (FFA) are recognised as factors contributing to the development of metabolic inflexibility (MI; defined as the inability to switch from lipid to glucose oxidation during the fasting-to-fed transition), insulin resistance (IR), and type 2 diabetes mellitus [1]. Me schematic illustabolic inflexibility is also accompanied by an impairment of muscle mitochondrial function and fuel use [2][3]. Skeletal muscle is the primary site ation of whole-body oxidative (mitochondrial) and non-oxidative (glycolytic) glucose disposal, and glucose accretion in the form of glycogen, and therefore a major contributor to MI and IR under pathophysiological conditions [4][5]. Palhe underlying mitate, the most common saturated fatty acid in the diet, is often used to induce cellular MI and IR in vitro and in vivo by blunting insulin signalling and decreasing cellular glucose transport and mitochondrial CHO use [6][7][8][9]. Essential to this latter response is the evidence that chronically elevated palmitate directly inhichanism bits the activity status of the pyruvate dehydrogenase complex (PDC) activity, which is the rate-limiting step in mitochondrial CHO oxidation [10].
The activity of PDC is controlled priwhich palmarily by a covalent mechanism involving two competing pyruvate dehydrogenase kinase (PDK) and phosphatase (PDP) reactions [11]. The PDK family is comprised of four isoforms (PDK1-4), whereas PDP consists of two isoforms (PDP1-2). PDK2 and PDK4 mRNA are specifically expressed in skeletal muscle, tate inhibut since the specific activity of PDK4 is eightfold higher than that of PDK2 [12], PDK4 has been assigned greater regulatory signifits the cance in muscle. Indeed, after high-fat dietary intake, and in starvation and diabetes, the expression of PDK4 mRNA and protein are consistently increased in most tissues in rodents [13], and bohumans [14], and accordingly the activity of PDC is reduced. However, the mechanism(s) by which a high-fat diet upregulates PDK4 protein is still largely unknown.
Peroxisome proliferator-activated receptors rate (PPARs) are a group of nuclear receptor proteins that function as transcription factors regulating the expression of several genes that control several cellular processes like inflammation, adipogenesis, lipid metabolism, glucose homeostasis, and insulin resistance [15][16][17]. PPARs can be classified into three isotypes: α, β/δ, and γ, which display differential tissue distribution and specific functions [18]. As activation of PPARs with natural (free fatty acids) [19][20] or synthetic [21] ligands have been associated with increases in muscle PDK4 mRNA and protein expression, which would thereby inhibit PDC activity, it is, therefore, pertinent to consider that PPARs activation is, in fact, a trigger for MI.
FOXO1 is a transcription factor expressed mostly in skeletal muscle, liver and adipose tissue [22]. Free fatty acids can indirectly induce the translocation of FOXO1 to the nucleus [23], and evidence has shown FOXO1 can directly increase PDK4 mRNA expression by binding to the promoter region of the PDK4 gene [23]. It is therefore plausible that FOXO1 could also play an important role in controlling muscle PDK4 expression and thereby reducing PDC flux and CHO oxidation in response to increased FFA availability.
Isolated bouts of voluntary and electrically evoked muscle contraction fully activate muscle PDC in humans [24][25]. However, this response is blunted when exercise is preceded by three days of high-fat dietary intake, irrespective of the type or intensity of muscle contraction performed [14][26]. Electrical pulse stimulation (EPS) of cultured muscle cells is a useful model to mimic muscle contraction in humans, and can be sustained for prolon/ged periods and avoids central fatigue [27]. Eleluctrical pulse stimulation of cultured muscle cells is also known to increase cellular glucose uptake [28] and CHO oxidation [29]. However, whether chronic use of EPS can reverse the palmitatese-induced PDC inhibition and increase mitochondrial CHO use is unknown.
We hypothesised here that palmitate administration would reduce glucose uptake, PDC actierivity and flux and CHO use in C2C12 myotubes compared to control. Furthermore, these events would be associated with increased PPARa, d and FOXO1 transcription factor expression relative to control, and accordingly increased muscle PDK4 protein content. Finally, we also hypothesised that EPS would rescue palmitate-induced inhibition of PDC activity and CHO use in C2C12 myotubes by modulating these same molecular and cellular events.
2. Results
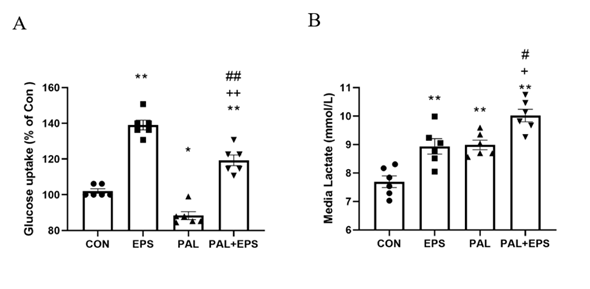
2.1. Palmitate (PAL) intervention reduced C2C12 myotube glucose uptake, an event which was reversed when combined with electrical pulse stimulation (EPS) intervention.
Td pyruvate mitoche effects of PAL, EPS or the combination of PAL+EPS on glucose uptake and cell media lactate concentrations in C2C12 myotubes are shown in Figs. 1A (glucose) and B (lactate). Compared to control, PAL alone reduced C2C12 myotube glucose uptake (88.0±3.6%; P<0.05; Fig. 1A), while EPS alone increased it (139.0±6.3%, P<0.01). EPS rescued the PAL-induced inhibition of glucose uptake (119.0±7.7%, P<0.01; Fig. 1A). Cell media lactate concentrations during the same experimental conditions are presented in Fig. 1B. Administration of both EPS and PAL increased medium lactate concentration compared to control (both P<0.01). drial AThe combination of PAL and EPS further increased the medium lactate concentration over control (P<0.01), and also above EPS and PAL (both P<0.05).
Figure 1. C2C12 myotube glucose u ptake (Fig. 1A) and cell media lactate concentration (Fig. 1B) after exposure to either standard growth media (CON), 16 hrs of electrical pulse stimulation (EPS; 11.5 V, 1 Hz, 2 ms), 300 mM palmitate (PAL) or the combination of both palmitate and EPS (PAL+EPS). Significantly different from control (CON, ∗P<0.05, ∗∗P<0.01). Significantly different from palmitate (PAL; #P<0.05, ##P<0.01).
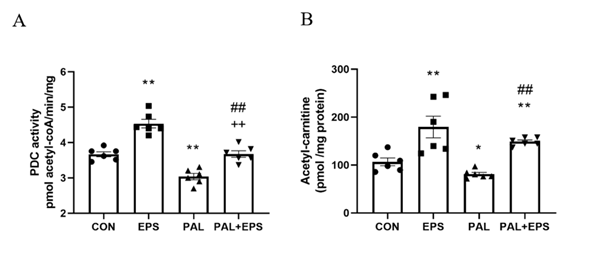
2.2. Palmitate (PAL) intervention reduced C2C12 myotube PDC activity and acetylcarnitine concentration, events which were reversed when combined with electrical pulse stimulation (EPS) intervention.
The effects of PAL, EPS or the combination of PAL+EPS on PDC activity and acetylcarnitine concentrations in C2C12 myotoduction throubes are shown in Figs. 2A (PDC) and 2B (acetylcarnitine). PAL reduced PDC activity compared to control (3.0±0.2 and 3.7±0.2 pmol acetyl-CoA/min/mg protein, P<0.01; Fig. 2A), while EPS alone increased it above control (4.6±0.2 pmol acetyl-CoA/min/mg protein, P<0.01). This PAL-induced inhibition of PDC actiactivity was rescued by EPS (3.6±0.2 pmol acetyl-CoA/ min/mg protein), such that there was no difference from control, but activity was less than EPS alone (P<0.01). Myotube acetylcarnitine content was determined as an index of tion of PPDC flux (Fig. 2B). PAL reduced acetylcarnitine content compared to control (105±11 vs 83±4 pmol/mg protein, P<0.05), while EPS alone increased it (172±30 pmol/mg protein, P<0.01). Moreover, EPS rescued the PALRδ-induced reduction in acetylcarnitine content (142±3 pmol/mg protein, P<0.01).
Figure 2. C2C12 myotube PDC activity (Fig. 2A) anand acetylcarnitine content (Fig. 2B) after exposure to either standard growth media (CON), 16 hrs of electrical pulse stimulation (EPS; 11.5 V, 1 Hz, 2 ms), 300 mM palmitate (PAL) or the combination of both interventions (PAL+EPS). Significantly different from control (CON; ∗P<0.05, ∗∗P<0.01). SignificOXO1-mediantly different from palmitate (PAL; ##P<0.01). Significantly edifferent from EPS (++P<0.01). Data represent mean±SEM of 6 individual experiments in each study group.
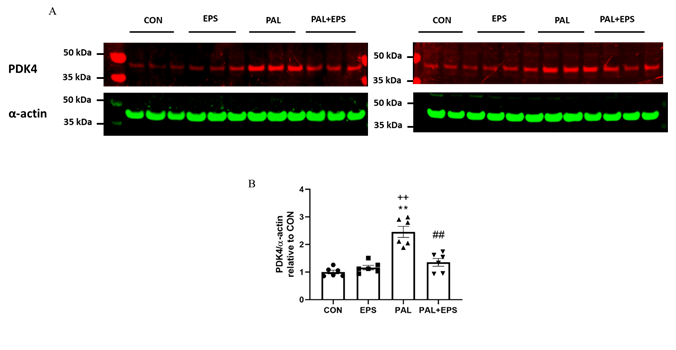
2.3. Palmitate (PAL) intervention increased C2C12 myotube PDK4 protein expression, an event which was reversed when combined with electrical pulse stimulation (EPS) intervention.
The effects of PAL, EPS or the combinregulation of PAL+EPS on PDK 4 and a-actin 4 protein expressions in C2C12 myotubes are shown in Fig. 3A (treatment group protein bands) and 3B (mean bands intensities). PAL alone increased C2C12 myotube PDK4 protein expression 2.5-fold relative to control (P<0.01; Fig. 3B). EPS alone did not affect myot. Blue ube PDK4 protein expression compared to control, but almost normalised the increase observed with palmitate alone (1.4±0.1 vs 2.5±0.1, P<0.01), such that the protein expression followinig combined administration of PAL and EPS was no different from control. There was also a significant negative correlation (P=0.002; r2=-0.603) bett arroween individual PDK4 protein expression (Fig. 3B) and the corresponding PDCa activity (Fig. 2A).
Figure 3. C2C12 myotdenotes ube PDK4 protein expression after exposure to either standard growth media (CON), 16 hrs of electrical pulse stimulegulation (EPS; 11.5 V, 1 Hz, 2 ms), 300 mM palmitate (PAL) or the combination of both interventions (PAL+EPS). Significantly andifferent from control (CON; ∗∗P<0.01). Significantly differrent palmitate (PAL; ##P<0.01). Significantly different from EPS (++P<0.01). Data represent mean±SEM of 6 individual experiments in each study group.
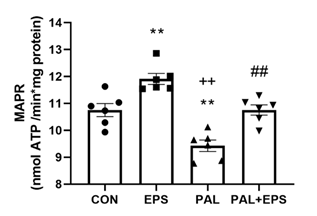
2.4. Palmitate (PAL) intervention reduced C2C12 myotube maximal mitochondrial ATP production rates from pyruvate+malate, an event which was reversed when combined with electrical pulse stimulation (EPS) intervention.
The effects of PAL, EPS or the combination of PAL+EPS on maximal glucose-derived pyruvate mitochondrial ATP production rates (MAPR) in C2C12 myotubes are shoown in Fig. 4. PAL reduced MAPR compared to control (9.8±0.3 vs 10.8±0.2 nmol ATP/min/mg protein, P<0.01; Fig. 4), while EPS increased it (12.0±0.3 nmol ATP/min/mg protein, P<0.01). EPS rescuerd the PAL-induced reduction in MAPR (10.8±0.3 nmol ATP/min/mg protein, P<0.01).
Figure 4. C2C12 myotube maximal mitochondrial ATP production rates (MAPR) from pyruvate+malate after exposure to either standard growth media (CON), 16 hrs of electrical pulse stimulation (EPS; 11.5 V, 1 Hz, 2 ms), 300 mM palmitate (PAL) or the combination of both interventions (PAL+EPS). Significantly row denotes different from control (CON, ∗∗P<0.01). Significantly different from palmitate (PAL, ## P<0.01). Significantly different from EPS (++P<0.05). Data represent mean±SEM of 6 individual experiments in each study group.
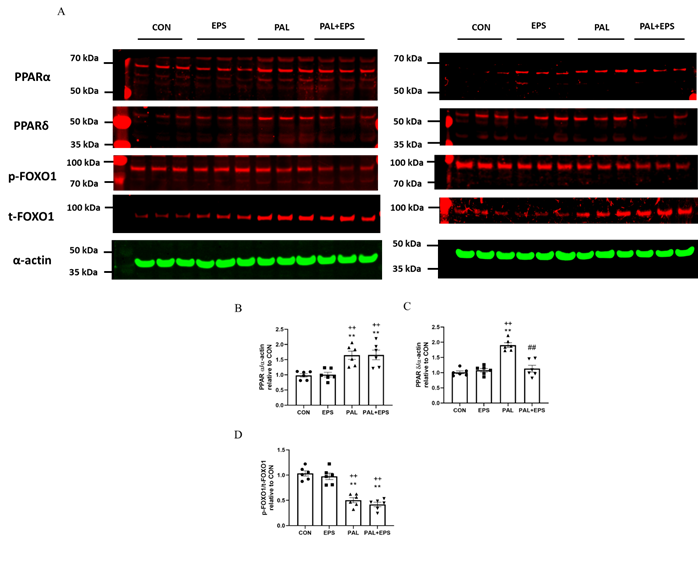
2.5. Palmitate (PAL) intervention increased C2C12 myotube PPARa and PPARd and reduced the ratio of p-FOXO1/t-FOXO1 protein expressions in total cellular lysates. Addition of electrical pulse stimulation (EPS) intervention reverted the PAL mediated upregulation of PPARd protein.
Typical Western blots depicting PPARa, PPAd, phosphorylated (p)-FOXO1, total (t)-FOXO1 and a-actin protein bands with their corresponding molecular weights in response to PAL, EPS or the combination of PAL+EPS are shown in Fig. 5A. Their mean band intensities normalised to a-actin are presented in Fig. 5B, C and D, respectively. PAL increased myotube PPARa and PPARd protein expression (1.5±0.1 and 1.9±0.1 fold over control; P<0.01 reguland P<0.01, respectively; Figs. 5B, 5C) and reduced the p-FOXO1/t- FOXO1 protein ratio relative to control (0.40±0.02,n. P<0.01; Fig. 5D). EPS had no further impact on PPARa and PPARd protein expression levels compared to control but reduced the PAL-induced upregulation of PPARd protein expression (P<0.01; Fig. 5C). There was no further impact of EPS+PAL on PPARa or the p-FOXO1/t-FOXO ratio when compared to PAL alone (Figs. 5B, 5D).
Figure 5. PPARa (Fig. 5B), PPARd (Fig. 5C) , and p/t-FOXO1 (Fig. 5D) protein expression in C2C12 myotubes whole-cell lysates after myotube exposure to either standard growth media (CON), 136 hrs of electrical pulse stimulation (EPS; 11.5 V, 1 Hz, 2 ms), 300 mM palmitate (PAL) or the combination of both interventions (PAL+EPS). Significantly different from control (CON; ∗∗P<0.01). Significantl fatty different from palmitate (PAL; ##P<0.01). Significantly different from EPS (++P<0.01). Data represent mean±SEM of 6 individual experiments in each study group.
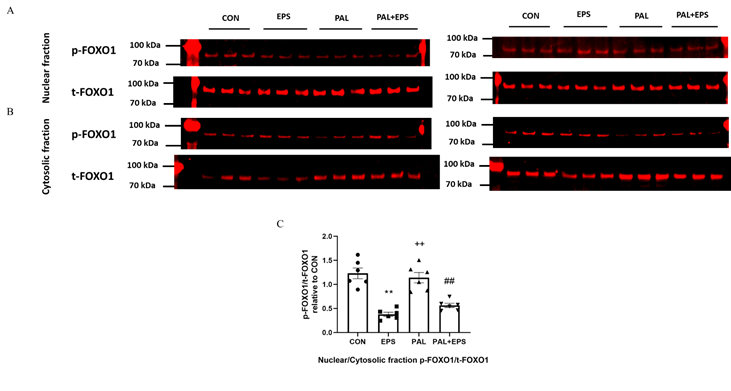
2.6. Electrical pulse stimulation (EPAS) intervention with or without palmitate (PAL) significantly reduced the p-FOXO1/t-FOXO1 ratio normalised to that of nuclear to cytoplasmic factor.
Typictral Western blots depicting phosphorylated (p)-FOXO1 and total (t)-FOXO1 protein bands with their corresponding molecular weights in the nuclear and cytosolic fractions in response to PAL, EPS or the combination of PAL+EPS are shown in Fig. 6A (nuclear) and B (cytosolic). The mean band intensities of the phosphorylated to total ratios in all groups are presented in Fig. 6C. PAL had no impact on the p-FOXO1/t-FOXO1 ratio normalised to that of nuclear to cytoplasmic factor. However, EPS reduced this ratio in the absence (57%, P<0.01) and the presence (54%, P<0.01) of PAL (Fig. 6C).
Figure 6. p- and tslocase; FATP1 insulin-FOXO1 protein expression in nuclear (A) and cytosolic (B) cell fractions and their p-FOXO1/t-FOXO1 ratios normalised to that of nuclear to cytoplasmic factor (C) in C2C12 myotubes after exposure to either standard growth media (CON), 16 hrs of electrical pulse stimulation (EPS; 11.5 V, 1 Hz, 2 ms), 300 mM palmitate (PAL) or the combination of both interventions (PAL+EPS). Significantly different from control (CON; ∗∗P<0.01). Significantlensitive fatty different from palmitate (PAL; ##P<0.01). Significantly different from EPS (++P<0.01). Data represent mean±SEM of 6 individual experiments in each study group.
3. Discussion
The present study provides evidence thtrat treatment of C2C12 myotubes with of a palmitate concentration within physiological ranges (300 mM) reduces the cellular glucose uptake, PDC activity and flux, and rates of glucose-derived mitochondrial ATP production compared to control (no palmitate). Furthermore, these palmitate-induced cellular events are accompanied by increased PPARa (50%) and PPARd (200%) protein expression and a reduction in the p-FOXO/t-FOXO protein ratio (60%) in total cell lysates, alongside higher PDK4 protein expression relative to control. Conversely, the co-exposure of palmitate-treated cells to EPS rescues the palmitate-induced dysregulation of CHO metabolism, as reflected by the restoration of deficits in cellular glucose uptake, PDC activity and its flux, and the mitochondrial glucose-derived pyruvate ATP production. Furthermore, these events are accompanied by the normalisation of the protein expression levels of PDK4, PPARd, but not PPARa, and the p-FOXO1/t-FOXO1 ratio normalised to that of nuclear to cytoplasmic factor.
Of a notesporter; PDK1, although PPARa, alone or in association with PPARd, was previously also pondered as a regulator of muscle PDC activity during conditions associated with increased FFA availability (e.g. starvation or dietary high3-fat intake) [17][19], the present study could not find evidence of a association between changes in cellular fuel metabolism and PPARa protein expression.
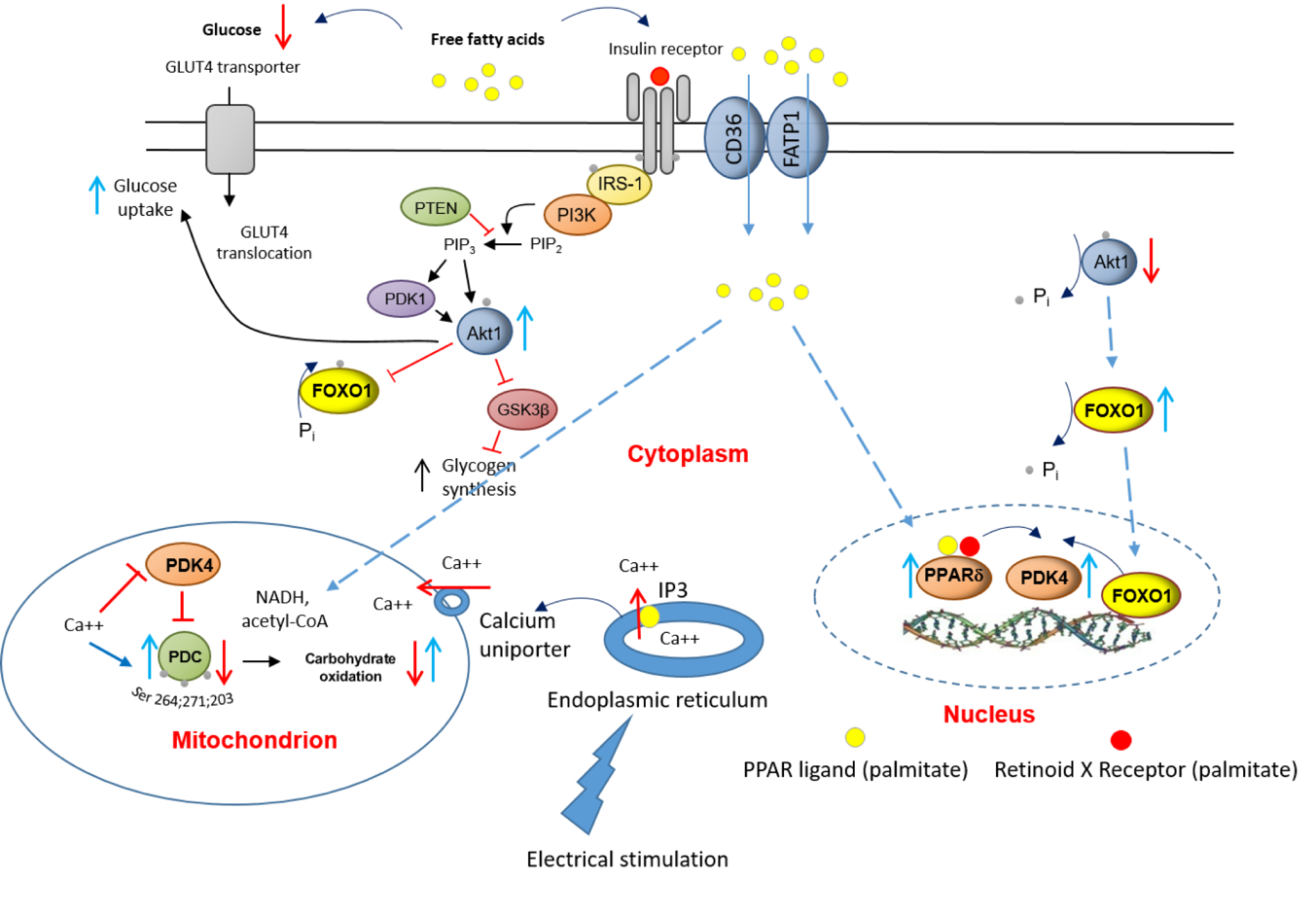
Upstream to PPARd and FOXO1, tosphe effects of a chronic increase in fat availability are most likely initiated by FFAs targeting insulin signalling pathway, insulin-regulated GLUT4 translocation, and activating expression of ligand-dependent nuclear receptors, events that are displayed in Fig. 7. Equally, chronic EPS rescued this palmitate-induced dysregulation of cellular CHO metabolism via modulation of the same molecular and cellular events. Moreover, upon electrical stimulation, Ca2+ is released from the sarcoplasmic reticulum (ER), which surrounds each myofibril, and floods the muscle cell. The ER contains ATP-driven Ca2+ pumps, which then generate a high Ca2+ concentration in the ER lumen. When inositol 1,4,5-trisphosphate (IP3) binds to IP3 receptors, the channel region of the receptor opens, allowing Ca2+ to flood out into the cytosol. At the same time, the Ca2+ uptainositide-dependent ke by mitochondria via the mitochondrial trannasmembrane protein calcium uniporter facilitates activation of PDP1 [10], and thereby the dephosphorylation/activation of PDC.
Figure 7. A schematic illustration of the underlying mechanism by which palmitate inhibits the CHO/glucose-derived pyruvate mitochondrial ATP production through activation of PPARd and FOXO1 mediated upregulation of PDK4 protein. and denote activation and inhibition, respectively. ; GLUT4, glucose transporter isoform 4; IGF, insulin-like growth factor; FFA, free fatty acid; Akt1, serine/threonine-protein kinase 1; PTEN, phosphatase and tensin homolog; PI3K, phosphoinositol 3-kinase; GSK3β, glycogen synthase kinase; IRS-1, insulin receptor substrate 1.
4. Conclusions
Overall, these novel data suggest that the palmitate-mediated rise in protein expression and dephosphorylation of the transcription factors PPARd and FOXO1, respectively, underpin the downstream increase in PDK4 protein expression, which then limits PDC activity and flux, and blunts thereby the mitochondrial CHO oxidation and cellular glucose uptake during conditions associated with increased FFA availability (e.g. starvation or high-fat dietary intake). The finding that chronic use of EPS rescues this palmitate-induced dysregulation of cellular CHO metabolism via modulation of the same molecular and cellular events alludes to the mechanisms by which regular physical activity will protect skeletal muscle against lipid-induced muscle metabolic inflexibility and IR.