Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Solmaz Abdolrahimzadeh and Version 2 by Conner Chen.
Choroidal neovascularizations are historically associated with exudative macular degeneration, nonetheless, they have been observed in nevus, melanoma, osteoma, and hemangioma involving the choroid and retina.
- choroidal neovascularization
- tumor
- choroid
1. Choroidal Neovascularization Mechanism in In-Vivo and In-Vitro Models
Choroidal neovascularization (CNV) is historically associated with exudative macular degeneration, which is characterized by the deposition of insoluble material in the retinal layers, choriocapillaris thinning, and alteration in Bruch’s membrane thickness. There is an accumulation of lipoproteins, which leads to retinal pigment epithelium (RPE) and photoreceptor atrophy, para-inflammation, and hypoxia. Together, they eventually lead to the secretion of vascular endothelial growth factor (VEGF) from RPE, photoreceptors, and immune cells, which promotes neovascularization [1][2][3][8,9,10]. Exudative age-related macular degeneration (AMD) is a consequence secondary to CNV [4][11] as shown in Figure 1.
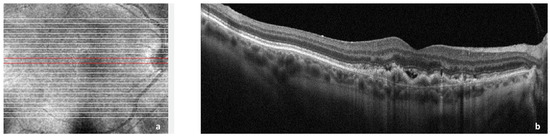
Figure 1. Spectral domain optical coherence tomography (SDOCT) of the macular area showing choroidal neovascularization (CNV) secondary to age-related macular degeneration. (a): Image of the macula showing overlying raster scan; (b): cross-sectional SDOCT scan showing retinal pigment epithelium (RPE) elevation due to CNV underlying the RPE and adjacent subretinal fluid.
The first classification of the different types and growth patterns was built in 1991 around fluorescein angiography (FA) leakage evidence dividing the neovascular membranes in occult or classic [5][12]. The occult neovascular membrane is an ingrowth of a neovascular complex initially from the choriocapillaris, into and within the sub-RPE space, that shows a stippled hyperfluorescence, which expands and becomes more evident only in the later phases of FA; classic neovascular membrane originates from the choroid and traverses Bruch’s membrane and the RPE; hence, it may be detected in the very early phase of FA [4][5][11,12]. In the following years, the histologic subtypes were classified as CNV type 1, where the CNV is located below the RPE, and CNV type 2, where the CNV is located above the RPE to describe occult and classic neovascularization, respectively [4][11]. Both are mainly composed of fibrovascular tissue. A combined subtype of type 1 and 2 was also identified [6][7][13,14].
2. Vascular Mechanisms in Choroidal Neovascularization
The first and leading hypothesis in 1987 was that all new vessels in CNV arise from pre-existing choroidal vasculature [8][31]. In the 1990s, bone marrow circulating progenitor cells were identified as contributors to adult vasculogenesis [9][10][32,33]. With the laser photocoagulation-induced injury to the choroid described above, researchers transplanted enhanced green fluorescent protein (EGFP)-expressing bone marrow cells from EGFP donor mice into laser-treated mice. Green fluorescent protein recruited cells (GFP+) were quantified in the choroidal vasculatures or in Bruch’s membrane injury sites, showing different levels of contribution in CNV [11][12][34,35]. The proportion of GFP+ cells contributing to lesion endothelial cells was observed to be dependent on the stage of CNV [13][14][36,37], and mobilized adult hematopoietic stem cells were observed to be able to form endothelial cells subsequently incorporated into choroidal neovasculature [15][38]. Similar hemangioblast activity was also observed in the murine model [16][39] and in humans. In the latter, the presence of bone marrow-derived progenitor was identified in excised CNV sections by tracing the AC133 marker of hematopoietic stem cells and bone marrow-derived progenitors [17][40].3. Molecular Mechanisms
VEGF is found in several isoforms, VEGF121, VEGF145, VEGF165, VEGF189, and VEGF206, and is a strong angiogenic molecule capable of stimulating proliferation, migration, and enhancing vascular permeability of endothelial cells [18][19][47,48]. Dysregulation of VEGF is known as one of the main steps in pathological angiogenesis [20][49]. In physiological conditions, VEGF and FGF2 are released by the RPE during fetal development to promote the development of the choriocapillaris [21][50]. Furthermore, VEGF is required for the formation of fenestrations in the choriocapillaris [22][51] in order to allow macromolecule transit in and out of choroidal circulation [21][50]. Under normal conditions, VEGF is kept at a basal level, but in pathological reactive conditions, such as CNV, VEGF levels are significantly increased [20][49]. The VEGF isoform that is predominantly involved in pathological angiogenesis is VEGF164/165 [23][52]. In addition to VEGFs’ stimulating role in angiogenesis, their elevated level in the RPE leads to barrier disruption, which could further increase neovascularization chances [22][51] as illustrated in Figure 2.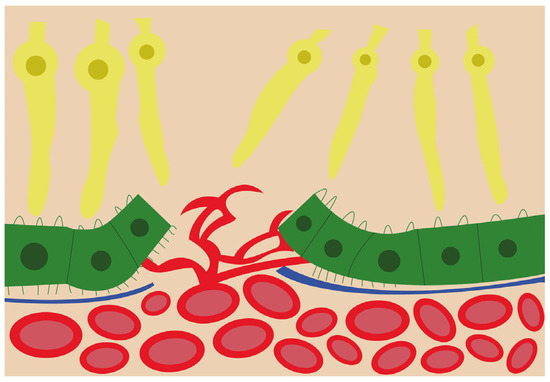
Figure 2. Schematic view of the choroidal neovascularization process. The new blood vessels from the choroid have breached the retinal pigment epithelium and are branching and invading the retina.
