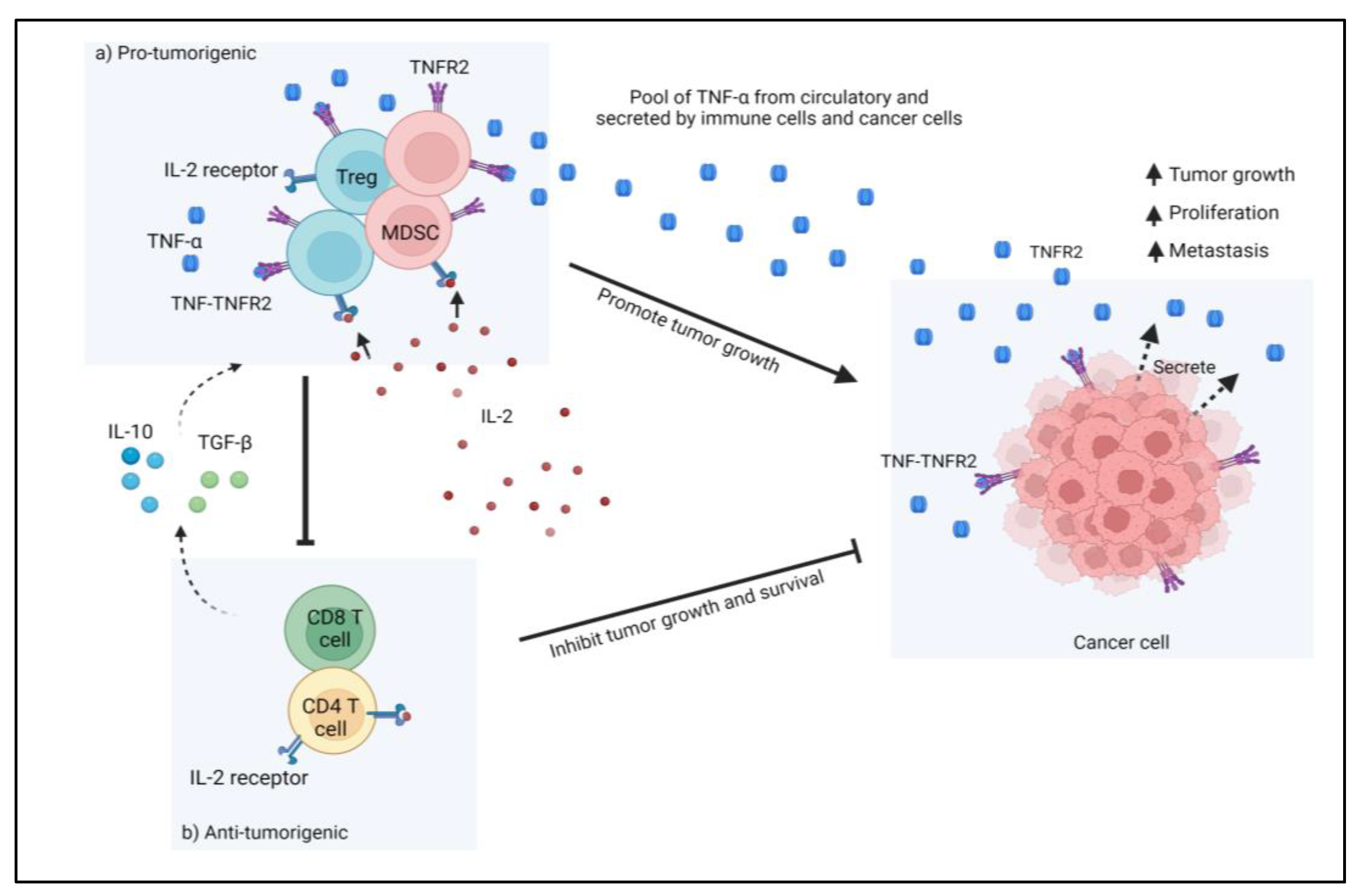
Colorectal cancer (CRC) represents one of the most common causes of death among cancers worldwide. Its incidence has been increasing among the young population. Many risk factors contribute to the development and progression of CRC and about 70% of them are sporadic. The CRC microenvironment is highly heterogeneous and represents a very complex immunosuppressive platform. Many cytokines and their receptors are vital participants in this immunosuppressive microenvironment. Tumor necrosis factors (TNFs) and TNF receptor 2 (TNFR2) are critical players in the development of CRC. TNFR2 was observed to have increased the immunosuppressive activity of CRC cells via regulatory T cells (T regs) and myeloid-derived suppressor cells (MDSC) in the CRC microenvironment.
- colorectal cancer
- TNFR2
- Treg cells
- MDSC
- TNF
- immunosuppressive
- tumor microenvironment
1. Introduction: Overview of Colorectal Cancer
2. Tumor Necrosis Factor Receptor 2 (TNFR2): An Outlook in CRC
3. Targeting TNFR2 in CRC Cancer
TNFR2 acts as both an oncogene and Treg- or MDSCs-cells inducer. Furthermore, its limited expression on other cell types renders this receptor a selective target for cancer treatment. In respect to CRC, TNFR2 is responsible for the promotion of proliferation and angiogenesis through several other mechanisms, such as by mediating progranulin [91][14] and STAT3 [33][11]. Currently, in a study with murine colon cancer models, the combination of anti-programmed death receptor-1 with new anti-TNFR2 led to complete tumor regression and elimination in more than 60% of the animals [44][15]. The mechanism behind this therapeutic potency is thought to involve the reduction of immunosuppressive Treg cells in the TME, thus increasing the ratio of the CD8+ effector cells to the Treg cells. The role of TNFR2 in CRC, however, is not only associated with its functional and preferential expression to Treg cells. Soluble TNFR2 (sTNFR2) reflects the upregulation of TNFR2 during inflammation, and the elevation of sTNFR2 was shown to increase the risk of CRC [92][16]. Additionally, higher sTNFR2 levels in patients with CRC are associated with higher mortality, although not CRC-specific mortality [93][17]. These roles of TNFR2 in the pathogenesis and progression of CRC render this receptor a potent target for CRC therapy. For cancers, including CRC, inhibition of TNFR2 or depletion of TNFR2-expressing cells can be achieved with the conventional antibody technology, and both in vitro and in vitro observations have been encouraging [44,57,89,94][15][18][19][20]. Although this approach could produce high-affinity anti-TNFR2, several limitations are of concern, including the unwanted FcγR-receptor side effects and the fact that it is only effective in the dominant manner of TNFR2 in the expansion of Treg cells [95][21]. TNFR2 is established to be preferentially expressed on Treg cells and important for their suppressive function; however, these characteristics are not exclusive to TNFR2 alone. Several other receptors, such as CD28 and CD25, as well as other TNFR receptors (GITR, OX40, 4-1BB), could also promote either the proliferation or the function of Treg cells, thus limiting the efficacy of targeting against any of these molecules. A better approach would be a system of targeting Treg cells as a whole by integrating all or most of their receptors. One such system is conceivably possible with the advent of nanotechnologies. Nanotechnologies have grown over the decades into a highly prospective tool in cancer therapy, where they are routinely used as drug and immune modulators and carriers that provide targeted delivery and prolonged accumulation in the organism [14][22]. Therefore, nanotechnologies may provide enhanced response rates and reduce the side effects that limit the full capacities of conventional drugs. Nanotechnology-based therapy can overcome the disadvantages of conventional therapy because of its small size, non-toxicity, biocompatibility, and distinct physicochemical properties [6]. Nanomaterials can be made comparable in size to biological macromolecules, making them ideal for cancer detection and treatment [6]. In addition, the advancement of nanotechnology-based medicinal approaches has resulted in significant improvements in the solubility of drugs as well as in their efficacy, bioavailability, and pharmacokinetics. As such, several nanomedicines have been approved by the FDA, such as liposomes [6] for cancer management, especially in the metastasis stage, and have been shown to fulfill their advantageous prospects, although there are still limited options existing for CRC [96][23].References
- Colorectal Cancer. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/10_8_9-Colorectum-fact-sheet.pdf (accessed on 7 November 2022).
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Colorectal Cancer; AICR: Washington, WA, USA, 2018.
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545.
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444.
- Vinchhi, P.; Patel, M.M. Triumph against cancer: Invading colorectal cancer with nanotechnology. Expert Opin. Drug Deliv. 2021, 18, 1169–1192.
- Abbas, A.K.; Lichtman, A.H.; Pober, J.S. B cell activation and antibody production. Cell. Mol. Immunol. 1991, 1, 243–267.
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T cells and immune tolerance. Cell 2008, 133, 775–787.
- Torrey, H.; Butterworth, J.; Mera, T.; Okubo, Y.; Wang, L.; Baum, D.; Defusco, A.; Plager, S.; Warden, S.; Huang, D.; et al. Targeting TNFR2 with antagonistic antibodies inhibits proliferation of ovarian cancer cells and tumor-associated Tregs. Sci. Signal. 2017, 10, eaaf8608.
- Zhao, T.; Li, H.; Liu, Z. Tumor necrosis factor receptor 2 promotes growth of colorectal cancer via the PI3K/AKT signaling pathway. Oncol. Lett. 2017, 13, 342–346.
- Hamilton, K.E.; Simmons, J.G.; Ding, S.; Van Landeghem, L.; Lund, P.K. Cytokine Induction of Tumor Necrosis Factor Receptor 2 Is Mediated by STAT3 in Colon Cancer CellsSTAT3-Dependent TNFR2 Induction in Colon Cancer Cell Lines. Mol. Cancer Res. 2011, 9, 1718–1731.
- Mizoguchi, E.; Mizoguchi, A.; Takedatsu, H.; Cario, E.; De Jong, Y.P.; Ooi, C.J.; Xavier, R.J.; Terhorst, C.; Podolsky, D.K.; Bhan, A.K. Role of tumor necrosis factor receptor 2 (TNFR2) in colonic epithelial hyperplasia and chronic intestinal inflammation in mice. Gastroenterology 2002, 122, 134–144.
- Ohue, Y.; Nishikawa, H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci. 2019, 110, 2080–2089.
- Yang, D.; Wang, L.L.; Dong, T.T.; Shen, Y.H.; Guo, X.S.; Liu, C.Y.; Liu, J.; Zhang, P.; Li, J.; Sun, Y.P. Progranulin promotes colorectal cancer proliferation and angiogenesis through TNFR2/Akt and ERK signaling pathways. Am. J Cancer Res. 2015, 5, 3085.
- Case, K.; Tran, L.; Yang, M.; Zheng, H.; Kuhtreiber, W.M.; Faustman, D.L. Tnfr2 blockade alone or in combination with PD-1 blockade shows therapeutic efficacy in murine cancer models. J. Leukoc. Biol. 2020, 107, 981–991.
- Kim, J.; Lee, J.; Oh, J.H.; Chang, H.J.; Sohn, D.K.; Shin, A.; Kim, J. Plasma inflammatory biomarkers and modifiable lifestyle factors associated with colorectal cancer risk. Clin. Nutr. 2020, 39, 2778–2785.
- Babic, A.; Shah, S.M.; Song, M.; Wu, K.; Meyerhardt, J.A.; Ogino, S.; Yuan, C.; Giovannucci, E.L.; Chan, A.T.; Stampfer, M.J.; et al. Soluble tumour necrosis factor receptor type II and survival in colorectal cancer. Br. J. Cancer. 2016, 114, 995–1002.
- Nie, H.; Zheng, Y.; Li, R.; Guo, T.B.; He, D.; Fang, L.; Liu, X.; Xiao, L.; Chen, X.; Wan, B.; et al. Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-α in rheumatoid arthritis. Nat. Med. 2013, 19, 322–328.
- Tam, E.M.; Fulton, R.B.; Sampson, J.F.; Muda, M.; Camblin, A.; Richards, J.; Koshkaryev, A.; Tang, J.; Kurella, V.; Jiao, Y.; et al. Antibody-mediated targeting of TNFR2 activates CD8+ T cells in mice and promotes antitumor immunity. Sci. Transl. Med. 2019, 11, eaax0720.
- Williams, G.S.; Mistry, B.; Guillard, S.; Ulrichsen, J.C.; Sandercock, A.M.; Wang, J.; González-Muñoz, A.; Parmentier, J.; Black, C.; Soden, J.; et al. Phenotypic screening reveals TNFR2 as a promising target for cancer immunotherapy. Oncotarget 2016, 7, 68278.
- Medler, J.; Wajant, H. Tumor necrosis factor receptor-2 (TNFR2): An overview of an emerging drug target. Expert Opin. Ther. Targets 2019, 23, 295–307.
- Gao, S.; Yang, D.; Fang, Y.; Lin, X.; Jin, X.; Wang, Q.; Wang, X.; Ke, L.; Shi, K. Engineering nanoparticles for targeted remodeling of the tumor microenvironment to improve cancer immunotherapy. Theranostics 2019, 9, 126.
- Ahmad, S.; Idris, R.A.M.; Wan Hanaffi, W.N.; Perumal, K.; Boer, J.C.; Plebanski, M.; Jaafar, J.; Lim, J.K.; Mohamud, R. Cancer nanomedicine and immune system—Interactions and challenges. Front. Nanotechnol. 2021, 3, 681305.
