Gram-negative bacteria are resistant to many commercialized antibiotics. The outer membrane (OM) of Gram-negative bacteria prevents the entry of such antibiotics. Outer membrane vesicles (OMV) are naturally released from the OM of Gram-negative bacteria for a range of purposes, including competition with other bacteria. OMV may carry, as part of the membrane or lumen, molecules with antibacterial activity. Such OMV can be exposed to and can fuse with the cell surface of different bacterial species.
- outer membrane vesicles
- antimicrobial activity
- Gram-negative bacteria
- Gram-positive bacteria
1. Introduction
2. OMV Biogenesis in Gram-Negative Bacteria
Gram-negative bacteria have an envelope comprising an OM and an inner membrane (IM) with a periplasmic space in between, which contains a layer of peptidoglycan (PG). In the OM there are lipopolysaccharides (LPS) linked covalently by the lipidic moiety and proteins bound as β-barrels, while in the IM the proteins are bound as α-helical [18]. The destabilization of ligations in a bacterium’s cell wall can lead to the detachment of the OM from the cell membrane. Consequently, the natural stabilization of the molecular charges allows the formation of the OMV [18,19][18][19]. OMV are nanostructures with size range between 20 and 250 nm that are secreted from the bacteria’s OM, being composed of phospholipids, LPS and outer membrane proteins (OMP) [19]. During formation, molecules such as nucleic acids and proteins from the periplasm and cytoplasm of the cells can be localized to the lumen of the vesicle, making it a vehicle for antibiotic resistance and virulence dissemination [18,20][18][20]. Some bacteria can also incorporate external antibiotics into vesicles, allowing isolation of antibiotics inside OMV and cell membrane stabilization [6]. The first step to the formation of OMV is the disruption of the connection OM-PG-IM without damage or loss of membrane integrity [18]. To explain the formation of OMV, three models have been created (Figure 1), which are not mutually exclusive: deficiency of lipoprotein (LPP) or its links in OM; increase of PG or other lipids residues; repulsion of negatively charged LPS [19].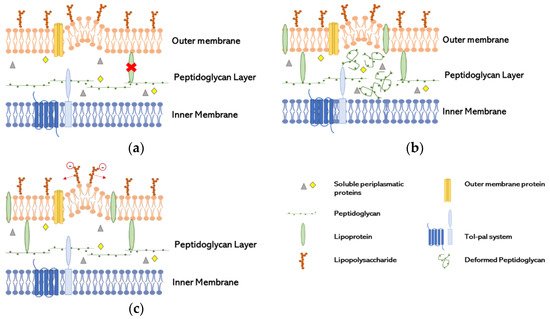
-
LPP links deficiency (Figure 1a): the presence of LPP in the unbound form has been found in OMV, indicating that the covalent links were broken, or their distribution was not homogenous, since the conversion of free-form LPP into bound form is reversible [21]. These characteristics seems to be induced by the non-proportional growth of the OM compared with the PG layer [22]. The relation of the lack of link between OmpA and PG has been proven to be essential to the production of OMV in Salmonella spp. [23].
-
Increase of misfolded PG (Figure 1b): Autolysins have a role in cleaving the covalent links of PG, resulting in cell wall remodeling. The lack of these enzymes increases the amount of peptides in periplasmatic space and other components leading to turgor pressure and therefore to OMV formation. Several studies explore the lack of autolysins to increase the concentration of proteins in the periplasmatic space and therefore converge to this model [24,25][24][25].
-
Repulsion of negatively charged LPS (Figure 1c): A study suggested that the repulsion of negatively charge B-band LPS in cells exposed to gentamicin, with great affinity to LPS, induce the release of OMV as a way of antibiotic resistance in which gentamicin was incorporated into OMV. That repulsion increased the production of vesicles in P. aeruginosa [6].
3. Antimicrobial Activity of OMV
The OMV antimicrobial activity is appealing for treatment purposes, especially against Gram-negative bacteria since it allows bridging with its cell wall. During OMV biogenesis, some molecules with antibacterial activity will be naturally included in the vesicle lumen. At the same time, several environmental inductors can enhance or contribute to the inclusion of those molecules into the OMV. In both cases, OMV can act as an antimicrobial agent.
3.1. OMV with Natural Antimicrobial Activity Cargo
The functions and roles of autolysin, also known as murein hydrolase [64[26][27],65], have being explored in Bacillus spp. [66][28]. Autolysins are usually PG-hydrolyzing endogenous enzymes that naturally lyse the peptide bridges in the PG layer [67][29], although there are some exceptions where it can have glycosidic activity [68][30]. There are several types of autolysins related with different mechanisms, such as protein and toxin secretion [69][31], flagellar formation [70[32][33],71], cell separation [72][34] and antibacterial activity associated to bacterial competition [12,73][12][35]. Its relation with vesicles and antimicrobial activity was observed for the first time in P. aeruginosa [6]. The presence of autolysins in OMV seem to be related with its normal location at the PG layer, being included in the cargo of the OMV during the bleb of the OM [6,64][6][26]. However, a recent study in Lysobacter spp. found that the distribution of the L5 enzyme, a bacteriolytic peptidase, may not be random, because it is only present in bacteria when this is exposed to a 30% sucrose medium. Thise study demonstrated that this autolysin is unevenly placed through the periplasmatic space, specifically where the vesiculation occurred and therefore L5 seems to be a factor in OMV biogenesis [74][36]. Hemolysin is another type of enzyme that is present in OMV from Gram-negative bacteria, such as P. aeruginosa and E. coli [6,54][6][37]. One hemolysin from P. aeruginosa has been shown to be responsible for co-regulation of protein secretion but also injection of toxin proteins into other Gram-negative bacteria, including E. coli [77][38], by type VI secretion system (T6SS) [78][39]. In addition, T6SS has been shown to be incorporated in OMV from P. aeruginosa [8]. The T6SS system allows the bacteria to compete through the delivery of toxins, that are capable of killing other bacteria [78][39].3.2. OMV with Loaded Antimicrobial Cargo
There are two main ways to incorporate antibiotics into OMV: the passive loading, where the addition of the antibiotics during bacterial growth is enough to produce antibiotic-carrying OMV (aOMV), and the active loading approach, where the antibiotics are forced to enter or coat the OMV or OM of bacteria, so it can be part of the produced OMV.
3.2.1. Passive Loading
Passive loading methods use diffusion by osmotic gradient but only for hydrophobic positively charged small molecules. These molecules may pass through the lipophilic membrane because of their opposite charges and their similar affinity to water [80][40]. In these methods, only the components of the medium or environmental characteristics are changed to destabilise the membrane and allow the entrance of antibiotics and other molecules [80,81][40][41]. Up to now, only antibiotics that have been demonstrated to pass through the cell wall of Gram-negative bacteria have been inserted into the OMV. Addition of gentamicin to P. aeruginosa cells showed the production of OMV-carrying gentamicin [6], which had antibacterial effects against both Gram-negative and Gram-positive bacteria species [12]. These gentamicin-carrying OMV showed antibacterial activity against P. aeruginosa 8803, which has a permeability-type resistance to gentamicin [12], highlighting the potential of OMV antimicrobial delivery to overcome resistance. More recently, different antibiotics, such as ceftriaxone, amikacin, azithromycin, ampicillin and levofloxacin, were loaded into A. baumannii OMV, which showed antibacterial effects against enterotoxigenic E. coli, Klebsiella pneumoniae and P. aeruginosa without toxic activity in mice [82][42].3.2.2. Active Loading
The active loading methods consist of forcing the entrance of the molecules into the vesicles, normally by physically damaging the cells or the vesicles. There are three main methods for active loading: electroporation, sonication, and extrusion, and they are already commonly used to produce vesicles from animal cells such as exosomes. Electroporation is an electro-physical method that uses electron impulses to rearrange the OM of the cell with the consequent creation of pores [34,83][43][44]. This method is typically used to make cell transfections of DNA, RNA and proteins, however its ability to translocate large molecules leads to an instable cell wall and therefore more cell death by lysis and less efficacy [34][43]. This method has been successfully used to insert small interference RNA into OMV from E. coli [35][45]. Sonication uses ultrasound to compress and decompress cells in order to compromise membrane stability, followed by a second sonication to assemble the membrane fragments and allow the incorporation of external molecules [36][46]. Mild sonication has been used successfully to induce paclitaxel loading into exosomes from macrophages to treat cancer cells and also to load small RNA into extracellular vesicles from different cell lines [37,85][47][48]. This method has already been used in Haemophilus parasuis to induce OMV production, however the protein content changed when compared to the natural OMV [86][49]. As far as we know, tThe use of sonication in bacterial studies is mostly used to induce cell lysis, and for detection of biofilms; studies related to OMV loading with sonication have not yet been performed [86,87,88,89][49][50][51][52]. Extrusion involves the mixture of cells and antibiotics added to a syringe extruder and then forced to pass through a porous membrane, under controlled temperature. The hydrostatic fluid pressure will disrupt the membrane, by increasing the axial tension, and allow the drug entrance at the same time that vesicles are formed; however, variation of size and zeta potential can occur [9,38][9][53]. Despite the yield in loading and forming vesicles with this method is high, the vesicles may not be homogenous and its impact in protein membrane structures is not clear, though higher pressures may lead to protein damage and cell death [38,39][53][54].4. Conclusions
OMV from Gram-negative bacteria have innate antibacterial activity due to the incorporation of several enzymes such as lysins. The incorporation of non-natural molecules into OMV with additional antibacterial effect has been shown. This suggests that a delivery system could be developed to overcome the OM barrier of the Gram-negative bacteria and that bacterial OMV can be used to deliver antibiotics to targeted populations of Gram-negative and -positive pathogenic bacteria. The OMV antimicrobial effect will depend on their cargo and the bacterial species targeted, as well as the resistance mechanism present in the target bacterial cells. The repurposing of antibiotics that are ineffective due to the permeability barrier of the cell wall of bacteria may also be possible with the use of OMV. The industrial production of OMV can be enhanced by changing the growth conditions or through different techniques that force cell damage; an alternative to OMV production with active lumen content is coating of the OMV with nanoparticles. However, several technical challenges that hamper the use of OMV remain, such as the types of MV isolated, the purification yield, and the optimal technique to produce vesicles with desired lumen content, especially hydrophilic molecules which cannot enter by passive loading. The reduction of the LPS toxicity is another point that needs to be optimized in order to reduce the immunogenic potential of OMV; for instance, the engineering of strains with altered LPS [105][55] might be a solution to produce OMV with reduced cytotoxicity. There is also still the need to better understand the target populations, including the specifics of the interaction between OMV and the target as well as subsequent host cell reactions. Overall, despite the challenges that must still be overcomed, OMV represent a cost-effective and safe drug delivery tool, representing a promising alternative for the treatment of bacterial infections caused by antibiotic-resistant bacteria and for the repurposing of antibiotics that are not usually effective against Gram-negative bacteria.References
- Garaeva, L.; Kamyshinsky, R.; Kil, Y.; Varfolomeeva, E.; Verlov, N.; Komarova, E.; Garmay, Y.; Landa, S.; Burdakov, V.; Myasnikov, A.; et al. Delivery of Functional Exogenous Proteins by Plant-Derived Vesicles to Human Cells in Vitro. Sci. Rep. 2021, 11, 6489.
- Fiocca, R.; Necchi, V.; Sommi, P.; Ricci, V.; Telford, J.; Cover, T.L.; Solcia, E. Release of Helicobacter Pylori Vacuolating Cytotoxin by Both a Specific Secretion Pathway and Budding of Outer Membrane Vesicles. Uptake of Released Toxin and Vesicles by Gastric Epithelium. J. Pathol. 1999, 188, 220–226.
- Marchant, P.; Carreño, A.; Vivanco, E.; Silva, A.; Nevermann, J.; Otero, C.; Araya, E.; Gil, F.; Calderón, I.L.; Fuentes, J.A. “One for All”: Functional Transfer of OMV-Mediated Polymyxin B Resistance From Salmonella Enterica Sv. Typhi ΔtolR and ΔdegS to Susceptible Bacteria. Front. Microbiol. 2021, 12, 1–15.
- Shima, T.; Muraoka, T.; Hamada, T.; Morita, M.; Takagi, M.; Fukuoka, H.; Inoue, Y.; Sagawa, T.; Ishijima, A.; Omata, Y.; et al. Micrometer-Size Vesicle Formation Triggered by UV Light. Langmuir 2014, 30, 7289–7295.
- Redeker, C.; Briscoe, W.H. Interactions between Mutant Bacterial Lipopolysaccharide (LPS-Ra) Surface Layers: Surface Vesicles, Membrane Fusion, and Effect of Ca2+ and Temperature. Langmuir 2019, 35, 15739–15750.
- Kadurugamuwa, J.L.; Beveridge, T.J. Virulence Factors Are Released from Pseudomonas Aeruginosa in Association with Membrane Vesicles during Normal Growth and Exposure to Gentamicin: A Novel Mechanism of Enzyme Secretion. J. Bacteriol. 1995, 177, 3998–4008.
- Chatterjee, S.; Mondal, A.; Mitra, S.; Basu, S. Acinetobacter Baumannii Transfers the BlaNDM-1 Gene via Outer Membrane Vesicles. J. Antimicrob. Chemother. 2017, 72, 2201–2207.
- Lin, J.; Zhang, W.; Cheng, J.; Yang, X.; Zhu, K.; Wang, Y.; Wei, G.; Qian, P.-Y.; Luo, Z.-Q.; Shen, X. A Pseudomonas T6SS Effector Recruits PQS-Containing Outer Membrane Vesicles for Iron Acquisition. Nat. Commun. 2017, 8, 14888.
- Fuhrmann, G.; Serio, A.; Mazo, M.; Nair, R.; Stevens, M.M. Active Loading into Extracellular Vesicles Significantly Improves the Cellular Uptake and Photodynamic Effect of Porphyrins. J. Control. Release 2015, 205, 35–44.
- Michel, L.V.; Gallardo, L.; Konovalova, A.; Bauer, M.; Jackson, N.; Zavorin, M.; McNamara, C.; Pierce, J.; Cheng, S.; Snyder, E.; et al. Ampicillin Triggers the Release of Pal in Toxic Vesicles from Escherichia Coli. Int. J. Antimicrob. Agents 2020, 56, 106163.
- Marion, C.R.; Lee, J.; Sharma, L.; Park, K.; Lee, C.; Liu, W.; Liu, P.; Feng, J.; Gho, Y.S.; Dela Cruz, C.S. Toll-Like Receptors 2 and 4 Modulate Pulmonary Inflammation and Host Factors Mediated by Outer Membrane Vesicles Derived from Acinetobacter Baumannii. Infect. Immun. 2019, 87, e00243-19.
- Kadurugamuwa, J.L.; Beveridge, T.J. Bacteriolytic Effect of Membrane Vesicles from Pseudomonas Aeruginosa on Other Bacteria Including Pathogens: Conceptually New Antibiotics. J. Bacteriol. 1996, 178, 2767–2774.
- Toyofuku, M.; Nomura, N.; Eberl, L. Types and Origins of Bacterial Membrane Vesicles. Nat. Rev. Microbiol. 2019, 17, 13–24.
- Domingues, S.; Nielsen, K.M. Membrane Vesicles and Horizontal Gene Transfer in Prokaryotes. Curr. Opin. Microbiol. 2017, 38, 16–21.
- Domingues, S.; Rosário, N.; Ben Cheikh, H.; Da Silva, G.J. ISAba1 and Tn6168 Acquisition by Natural Transformation Leads to Third-Generation Cephalosporins Resistance in Acinetobacter Baumannii. Infect. Genet. Evol. 2018, 63, 13–16.
- Park, J.; Kim, M.; Shin, B.; Kang, M.; Yang, J.; Lee, T.K.; Park, W. A Novel Decoy Strategy for Polymyxin Resistance in Acinetobacter Baumannii. Elife 2021, 10, 1–29.
- Collins, S.M.; Brown, A.C. Bacterial Outer Membrane Vesicles as Antibiotic Delivery Vehicles. Front. Immunol. 2021, 12, 733064.
- Schwechheimer, C.; Sullivan, C.J.; Kuehn, M.J. Envelope Control of Outer Membrane Vesicle Production in Gram-Negative Bacteria. Biochemistry 2013, 52, 3031–3040.
- Avila-Calderón, E.D.; Ruiz-Palma, M.D.S.; Aguilera-Arreola, M.G.; Velázquez-Guadarrama, N.; Ruiz, E.A.; Gomez-Lunar, Z.; Witonsky, S.; Contreras-Rodríguez, A. Outer Membrane Vesicles of Gram-Negative Bacteria: An Outlook on Biogenesis. Front. Microbiol. 2021, 12, 557902.
- Lee, E.-Y.; Bang, J.Y.; Park, G.W.; Choi, D.-S.; Kang, J.S.; Kim, H.-J.; Park, K.-S.; Lee, J.-O.; Kim, Y.-K.; Kwon, K.-H.; et al. Global Proteomic Profiling of Native Outer Membrane Vesicles Derived from Escherichia Coli. Proteomics 2007, 7, 3143–3153.
- Hoekstra, D.; van der Laan, J.W.; de Leij, L.; Witholt, B. Release of Outer Membrane Fragments from Normally Growing Escherichia Coli. Biochim. Biophys. Acta 1976, 455, 889–899.
- Wensink, J.; Witholt, B. Outer-Membrane Vesicles Released by Normally Growing Escherichia Coli Contain Very Little Lipoprotein. Eur. J. Biochem. 1981, 116, 331–335.
- Deatherage, B.L.; Lara, J.C.; Bergsbaken, T.; Rassoulian Barrett, S.L.; Lara, S.; Cookson, B.T. Biogenesis of Bacterial Membrane Vesicles. Mol. Microbiol. 2009, 72, 1395–1407.
- Hayashi, J.; Hamada, N.; Kuramitsu, H.K. The Autolysin of Porphyromonas Gingivalis Is Involved in Outer Membrane Vesicle Release. FEMS Microbiol. Lett. 2002, 216, 217–222.
- Zhou, L.; Srisatjaluk, R.; Justus, D.E.; Doyle, R.J. On the Origin of Membrane Vesicles in Gram-Negative Bacteria. FEMS Microbiol. Lett. 1998, 163, 223–228.
- Li, Z.; Clarke, A.J.; Beveridge, T.J. A Major Autolysin of Pseudomonas Aeruginosa: Subcellular Distribution, Potential Role in Cell Growth and Division and Secretion in Surface Membrane Vesicles. J. Bacteriol. 1996, 178, 2479–2488.
- Evans, A.G.L.; Davey, H.M.; Cookson, A.; Currinn, H.; Cooke-Fox, G.; Stanczyk, P.J.; Whitworth, D.E. Predatory Activity of Myxococcus Xanthus Outer-Membrane Vesicles and Properties of Their Hydrolase Cargo. Microbiology 2012, 158, 2742–2752.
- HOSODA, J.; NOMURA, M. Nature of the Primary Action of the Autolysin of Bacillus Subtilis. J. Bacteriol. 1956, 72, 573–581.
- Ghuysen, J.M. Use of Bacteriolytic Enzymes in Determination of Wall Structure and Their Role in Cell Metabolism. Bacteriol. Rev. 1968, 32, 425–464.
- Oshida, T.; Sugai, M.; Komatsuzawa, H.; Hong, Y.M.; Suginaka, H.; Tomasz, A. A Staphylococcus Aureus Autolysin That Has an N-Acetylmuramoyl-L-Alanine Amidase Domain and an Endo-Beta-N-Acetylglucosaminidase Domain: Cloning, Sequence Analysis, and Characterization. Proc. Natl. Acad. Sci. USA 1995, 92, 285–289.
- Hodak, H.; Galán, J.E. A Salmonella Typhi Homologue of Bacteriophage Muramidases Controls Typhoid Toxin Secretion. EMBO Rep. 2013, 14, 95–102.
- Scheurwater, E.; Reid, C.W.; Clarke, A.J. Lytic Transglycosylases: Bacterial Space-Making Autolysins. Int. J. Biochem. Cell Biol. 2008, 40, 586–591.
- Ishida, S.T.; Ishida, T. Zinc-Induced Immune Anti-Bacterial Vaccine Activity in Bacterial Cell Walls against Gram-Positive and Gram-Negative Bacteria. Proc. ARC J. Immunol. Vaccines 2019, 4, 10–19.
- Yamada, S.; Sugai, M.; Komatsuzawa, H.; Nakashima, S.; Oshida, T.; Matsumoto, A.; Suginaka, H. An Autolysin Ring Associated with Cell Separation of Staphylococcus Aureus. J. Bacteriol. 1996, 178, 1565–1571.
- Li, Z.; Clarke, A.J.; Beveridge, T.J. Gram-Negative Bacteria Produce Membrane Vesicles Which Are Capable of Killing Other Bacteria. J. Bacteriol. 1998, 180, 5478–5483.
- Kudryakova, I.V.; Suzina, N.E.; Vasilyeva, N.V. Biogenesis of Lysobacter Sp. XL1 Vesicles. FEMS Microbiol. Lett. 2015, 362, fnv137.
- Chen, S.; Yang, D.; Wen, Y.; Jiang, Z.; Zhang, L.; Jiang, J.; Chen, Y.; Hu, T.; Wang, Q.; Zhang, Y.; et al. Dysregulated Hemolysin Liberates Bacterial Outer Membrane Vesicles for Cytosolic Lipopolysaccharide Sensing. PLoS Pathog. 2018, 14, e1007240.
- Hood, R.D.; Singh, P.; Hsu, F.; Güvener, T.; Carl, M.A.; Trinidad, R.R.S.; Silverman, J.M.; Ohlson, B.B.; Hicks, K.G.; Plemel, R.L.; et al. A Type VI Secretion System of Pseudomonas Aeruginosa Targets a Toxin to Bacteria. Cell Host Microbe 2010, 7, 25–37.
- Russell, A.B.; Hood, R.D.; Bui, N.K.; LeRoux, M.; Vollmer, W.; Mougous, J.D. Type VI Secretion Delivers Bacteriolytic Effectors to Target Cells. Nature 2011, 475, 343–347.
- Nikaido, H.; Vaara, M. Molecular Basis of Bacterial Outer Membrane Permeability. Microbiol. Rev. 1985, 49, 1–32.
- Blokhina, S.V.; Sharapova, A.V.; Ol’khovich, M.V.; Volkova, T.V.; Perlovich, G.L. Solubility, Lipophilicity and Membrane Permeability of Some Fluoroquinolone Antimicrobials. Eur. J. Pharm. Sci. 2016, 93, 29–37.
- Huang, W.; Zhang, Q.; Li, W.; Yuan, M.; Zhou, J.; Hua, L.; Chen, Y.; Ye, C.; Ma, Y. Development of Novel Nanoantibiotics Using an Outer Membrane Vesicle-Based Drug Efflux Mechanism. J. Control. Release 2020, 317, 1–22.
- Weaver, J.C. Electroporation Theory. Concepts and Mechanisms. Methods Mol. Biol. 1995, 47, 1–26.
- Stankevic, V.; Simonis, P.; Zurauskiene, N.; Stirke, A.; Dervinis, A.; Bleizgys, V.; Kersulis, S.; Balevicius, S. Compact Square-Wave Pulse Electroporator with Controlled Electroporation Efficiency and Cell Viability. Symmetry 2020, 12, 412.
- Gujrati, V.; Kim, S.; Kim, S.-H.; Min, J.J.; Choy, H.E.; Kim, S.C.; Jon, S. Bioengineered Bacterial Outer Membrane Vesicles as Cell-Specific Drug-Delivery Vehicles for Cancer Therapy. ACS Nano 2014, 8, 1525–1537.
- Pick, U. Liposomes with a Large Trapping Capacity Prepared by Freezing and Thawing of Sonicated Phospholipid Mixtures. Arch. Biochem. Biophys. 1981, 212, 186–194.
- Lamichhane, T.N.; Jeyaram, A.; Patel, D.B.; Parajuli, B.; Livingston, N.K.; Arumugasaamy, N.; Schardt, J.S.; Jay, S.M. Oncogene Knockdown via Active Loading of Small RNAs into Extracellular Vesicles by Sonication. Cell. Mol. Bioeng. 2016, 9, 315–324.
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of Exosome-Encapsulated Paclitaxel to Overcome MDR in Cancer Cells. Nanomedicine 2016, 12, 655–664.
- McCaig, W.D.; Loving, C.L.; Hughes, H.R.; Brockmeier, S.L. Characterization and Vaccine Potential of Outer Membrane Vesicles Produced by Haemophilus Parasuis. PLoS ONE 2016, 11, e0149132.
- Li, K.; Yuan, X.-X.; Sun, H.-M.; Zhao, L.-S.; Tang, R.; Chen, Z.-H.; Qin, Q.-L.; Chen, X.-L.; Zhang, Y.-Z.; Su, H.-N. Atomic Force Microscopy of Side Wall and Septa Peptidoglycan From Bacillus Subtilis Reveals an Architectural Remodeling During Growth. Front. Microbiol. 2018, 9, 620.
- Foladori, P.; Laura, B.; Gianni, A.; Giuliano, Z. Effects of Sonication on Bacteria Viability in Wastewater Treatment Plants Evaluated by Flow Cytometry--Fecal Indicators, Wastewater and Activated Sludge. Water Res. 2007, 41, 235–243.
- Gomes, A.; van Oosten, M.; Bijker, K.L.B.; Boiten, K.E.; Salomon, E.N.; Rosema, S.; Rossen, J.W.A.; Natour, E.; Douglas, Y.L.; Kampinga, G.A.; et al. Sonication of Heart Valves Detects More Bacteria in Infective Endocarditis. Sci. Rep. 2018, 8, 12967.
- Zhang, Y.-F.; Shi, J.-B.; Li, C. Small Extracellular Vesicle Loading Systems in Cancer Therapy: Current Status and the Way Forward. Cytotherapy 2019, 21, 1122–1136.
- Gayán, E.; Govers, S.K.; Aertsen, A. Impact of High Hydrostatic Pressure on Bacterial Proteostasis. Biophys. Chem. 2017, 231, 3–9.
- Sharif, E.; Eftekhari, Z.; Mohit, E. The Effect of Growth Stage and Isolation Method on Properties of ClearColiTM Outer Membrane Vesicles (OMVs). Curr. Microbiol. 2021, 78, 1602–1614.
