Redox regulation participates in the control of various aspects of metabolism. Reactive oxygen and nitrogen species participate in many reactions under physiological conditions. When these species overcome the antioxidant defense system, a distressed status emerges, increasing biomolecular damage and leading to functional alterations. Air pollution is one of the exogenous sources of reactive oxygen and nitrogen species. Ambient airborne particulate matter (PM) is important because of its complex composition, which includes transition metals and organic compounds. Once in contact with the lungs’ epithelium, PM components initiate the synthesis of inflammatory mediators, macrophage activation, modulation of gene expression, and the activation of transcription factors, which are all related to the physiopathology of chronic respiratory diseases, including cancer.
- respiratory diseases
- air pollution
1. Introduction
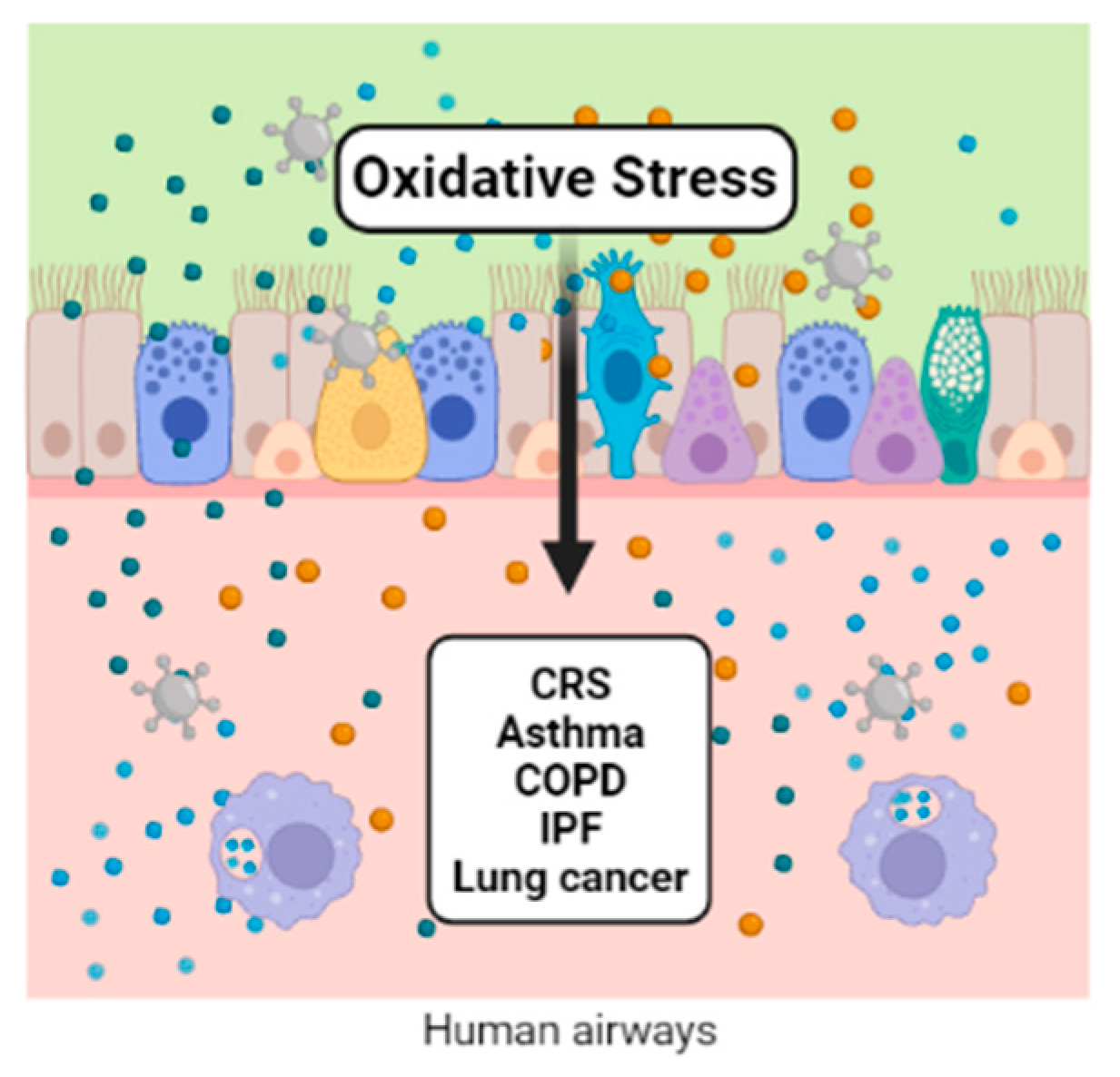
2. Chronic Rhinosinusitis (CRS) and Nasal Polyps (NP)
3. Asthma
Asthma is a complex condition that is heterogeneous and is characterized by the critical role of chronic airway inflammation and oxidative stress. The eosinophils, lymphocytes, neutrophils, and mast cells generate inflammatory mediators and ROS/RNS that negatively affect the redox balance [[40],[41]]. Furthermore, these are the basis for identifying the actual Type 2 high and Type 2 low phenotypes [[42]]. In Type 2 asthma patients, environmental factors favor the release of alarmins from the respiratory epithelium, which induces the differentiation of naïve T cells into Th2 cells. Damaged cells release interleukins such as IL−6, IL-1β, nitric oxide (NO•), prostaglandin E2 (PGE2), and tumor necrosis factor α (TNFα); the principal marker in these patients is the sputum eosinophilia [[43]]. T2-low asthma patients are characterized by sputum neutrophilia secondary to the activation of the NLRP3 inflammasome and elevated IL-1β; the activation of Th1 and/or Th17 cells associated with the imbalance of Th17/Treg cells seems to play an essential role in the pathology of asthma [[44]]. The response to the combination of Th1, Th2, and Th17 and genetic predisposition induce permanent structural changes in T2-high and T2-low asthma patients [[45],[46]]. The process of airway remodeling is driven by subepithelial fibrosis, thickening of the sub-basement membrane, increased airway smooth muscle mass, angiogenesis, and mucous gland hyperplasia [[45]]. An imbalance in the airway-reducing state is a determinant of the initiation and severity of asthma. The ability of an individual to ward off oxidative lung damage depends partly on their endogenous antioxidant systems and exogenous antioxidant intake [[27]]. Several groups have shown that the levels of enzymatic antioxidants such as SOD, catalase, and glutathione peroxidases, as well as heme oxygenase-1 (HO-1), thioredoxins, peroxiredoxins, and glutaredoxins, are decreased in the bronchoalveolar lavage, sputum, and serum of asthmatic patients [[40],[47],[48]]. Some factors increase the risk of the development of asthma. Among these, regular exposure through inhalation to oxidants derived from outdoor and indoor ambient air pollutants is on the list of factors that contributes to the progression of the disease [[49],[50],[51]]. Since the relationship between oxidative stress and the inflammatory response depends on each other, and genetic predisposition could modify their balance, there is interest in the role of the inflammatory process as an activator of oxidative stress [[52]]. Signaling pathways involving the inflammatory process and the oxidative response associated with the development of asthma are a current matter of evaluation. For example, the adenosine 5’ monophosphate-activated protein kinase (AMPK)/sirtuin 1 (Sirt1) and Nrf2/HO-1 pathway [[53]] and the nitrogen-activated protein kinase (MAPK) pathway that includes extracellular signal-regulated kinases (ERKs), c-Jun N-terminal kinase (JNK), and p38 [[54]] have been evaluated. Nrf2 potentiates the activity of the antioxidant response element (ARE) that synthesizes antioxidant proteins such as HO-1 [[55]]. Multiple phytochemicals involved in the immune response activate the Nrf2/HO-1 signaling axis. In this sense, the Nrf2/HO-1, NF-κB, and MAPK pathways are relevant therapeutic molecular targets in asthma [[56],[57],[58],[59],[60]]. The methodologies used to identify the molecular biomarkers associated with respiratory diseases are varied and range from the use of proteomics platforms to the use of real-time PCR. In 2021, Suzuki et al. evaluated the plasma proteome using an aptamer-base affinity proteomic platform (SOMAscan®) in 34 subjects with stable COPD and 51 subjects with asthma, detecting 1238 proteins within which stress markers were found, such as myeloperoxidase (MPO), heme oxygenase 2 (HMOX2), superoxide dismutase (Cu-Zn) (SOD1), peroxiredoxin-1 (PRDX1), and glutathione-S-transferase P1 (GSTP1) [[61]]. However, some markers are associated with oxidative stress-related cell damage, such as MDA, which can be measured by colorimetric techniques, high-performance liquid chromatography (HPLC), or LC/atmospheric pressure chemical ionization tandem mass spectrometry (LC/APCI–MS/MS). In asthmatic patients, the sputum measurement of MDA in the sputum discriminated between patients and controls with greater accuracy than the levels found in plasma, where it might be more difficult to evidence the redox imbalance due to comorbidities and lifestyle risk factors. In addition, 8-isoprostane and the oxidative DNA damage marker 8-oxo-7,8-dihydro-29-deoxyguanosine (8-OHdG) were also increased in the sputum from asthmatic patients compared with nonasthmatic controls in several studies [[62]]. The mitochondria are the organelles that contributes the most to the generation of reactive oxygen species, and its contribution to oxidative stress in asthma has also been evaluated. The mitochondria are also susceptible to oxidative stress; under such conditions, they undergo an adaptive response through mitochondrial biogenesis. In 2021, Carpagnano et al. determined that the mitochondrial DNA/nuclear DNA (mtDNA/nDNA) ratio was a marker of mitochondrial oxidative stress in the exhaled breath condensate (EBC) of 53 patients with severe asthma, 11 patients with mild to moderate asthma, and 12 healthy subjects. They found higher levels of exhaled mtDNA/nDNA in severe asthmatic patients compared with the mild-moderate and healthy controls; this may be useful for differentiating the asthma phenotypes [[63]]. It is crucial to take into account that the presence of oxidative stress is a factor that triggers asthma symptoms and contributes to the severity of the disease. Moreover, oxidative stress promotes corticosteroid insensitivity by disrupting glucocorticoid receptor (GR) signaling, leading to the sustained activation of proinflammatory pathways in immune cells and the airway’s structural cells [[64],[65]]. As already described in this section, many methodological strategies and various target molecules are related to oxidative stress. However, specific biomarkers with clinical applications in asthma have not yet been found.References
- Pruchniak, M.P.; Araźna, M.; Demkow, U. Biochemistry of Oxidative Stress BT—Advances in Clinical Science; Pokorski, M., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 9–19. ISBN 978-3-319-21497-9.
- Kruk, J.; Aboul-Enein, H.Y.; Kładna, A.; Bowser, J.E. Oxidative Stress in Biological Systems and Its Relation with Pathophysiological Functions: The Effect of Physical Activity on Cellular Redox Homeostasis. Free Radic. Res. 2019, 53, 497–521.
- Yang, S.; Lian, G. ROS and Diseases: Role in Metabolism and Energy Supply. Mol. Cell. Biochem. 2020, 467, 1–12.
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383.
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763.
- Mudway, I.S.; Kelly, F.J.; Holgate, S.T. Oxidative Stress in Air Pollution Research. Free Radic. Biol. Med. 2020, 151, 2–6.
- Sedaghat, A.R.; Kuan, E.C.; Scadding, G.K. Epidemiology of Chronic Rhinosinusitis: Prevalence and Risk Factors. J. Allergy Clin. Immunol. Pract. 2022, 10, 1395–1403.
- Patel, T.R.; Tajudeen, B.A.; Brown, H.; Gattuso, P.; LoSavio, P.; Papagiannopoulos, P.; Batra, P.S.; Mahdavinia, M. Association of Air Pollutant Exposure and Sinonasal Histopathology Findings in Chronic Rhinosinusitis. Am. J. Rhinol. Allergy 2021, 35, 761–767.
- Velasquez, N.; Gardiner, L.; Cheng, T.Z.; Moore, J.A.; Boudreau, R.M.; Presto, A.A.; Lee, S.E. Relationship between Socioeconomic Status, Exposure to Airborne Pollutants, and Chronic Rhinosinusitis Disease Severity. Int. Forum Allergy Rhinol. 2022, 12, 172–180.
- Velasquez, N.; Moore, J.A.; Boudreau, R.M.; Mady, L.J.; Lee, S.E. Association of Air Pollutants, Airborne Occupational Exposures, and Chronic Rhinosinusitis Disease Severity. Int. Forum Allergy Rhinol. 2020, 10, 175–182.
- Zheng, K.; Hao, J.; Xiao, L.; Wang, M.; Zhao, Y.; Fan, D.; Li, Y.; Wang, X.; Zhang, L. Expression of Nicotinamide Adenine Dinucleotide Phosphate Oxidase in Chronic Rhinosinusitis with Nasal Polyps. Int. Forum Allergy Rhinol. 2020, 10, 646–655.
- Mihalj, H.; Butković, J.; Tokić, S.; Štefanić, M.; Kizivat, T.; Bujak, M.; Baus Lončar, M.; Mihalj, M. Expression of Oxidative Stress and Inflammation-Related Genes in Nasal Mucosa and Nasal Polyps from Patients with Chronic Rhinosinusitis. Int. J. Mol. Sci. 2022, 23, 5521.
- Zorlu, M.E.; Uygur, K.K.; Yılmaz, N.S.; Demirel, Ö.Ö.; Aydil, U.; Kızıl, Y.; Uslu, S. Evaluation of Advanced Oxidation Protein Products (AOPP) and Superoxide Dismutase (SOD) Tissue Levels in Patients with Nasal Polyps. Indian J. Otolaryngol. Head Neck Surg. 2022.
- Alhawiti, N.M.; Al Mahri, S.; Aziz, M.A.; Malik, S.S.; Mohammad, S. TXNIP in Metabolic Regulation: Physiological Role and Therapeutic Outlook. Curr. Drug Targets 2017, 18, 1095–1103.
- Lin, H.; Ba, G.; Tang, R.; Li, M.; Li, Z.; Li, D.; Ye, H.; Zhang, W. Increased Expression of TXNIP Facilitates Oxidative Stress in Nasal Epithelial Cells of Patients With Chronic Rhinosinusitis With Nasal Polyps. Am. J. Rhinol. Allergy 2021, 35, 607–614.
- Ramanathan, M.J.; Tharakan, A.; Sidhaye, V.K.; Lane, A.P.; Biswal, S.; London, N.R.J. Disruption of Sinonasal Epithelial Nrf2 Enhances Susceptibility to Rhinosinusitis in a Mouse Model. Laryngoscope 2021, 131, 713–719.
- London, N.R.J.; Tharakan, A.; Mendiola, M.; Sussan, T.E.; Chen, M.; Dobzanski, A.; Lane, A.P.; Sidhaye, V.; Biswal, S.; Ramanathan, M.J. Deletion of Nrf2 Enhances Susceptibility to Eosinophilic Sinonasal Inflammation in a Murine Model of Rhinosinusitis. Int. Forum Allergy Rhinol. 2019, 9, 114–119.
- London, N.R.J.; Tharakan, A.; Lane, A.P.; Biswal, S.; Ramanathan, M.J. Nuclear Erythroid 2-Related Factor 2 Activation Inhibits House Dust Mite-Induced Sinonasal Epithelial Cell Barrier Dysfunction. Int. Forum Allergy Rhinol. 2017, 7, 536–541.
- Nishida, M.; Takeno, S.; Takemoto, K.; Takahara, D.; Hamamoto, T.; Ishino, T.; Kawasumi, T. Increased Tissue Expression of Lectin-Like Oxidized LDL Receptor-1 (LOX-1) Is Associated with Disease Severity in Chronic Rhinosinusitis with Nasal Polyps. Diagnostics 2020, 10, 246.
- Kawasumi, T.; Takeno, S.; Ishikawa, C.; Takahara, D.; Taruya, T.; Takemoto, K.; Hamamoto, T.; Ishino, T.; Ueda, T. The Functional Diversity of Nitric Oxide Synthase Isoforms in Human Nose and Paranasal Sinuses: Contrasting Pathophysiological Aspects in Nasal Allergy and Chronic Rhinosinusitis. Int. J. Mol. Sci. 2021, 22, 7561.
- Paoletti, G.; Casini, M.; Malvezzi, L.; Pirola, F.; Russo, E.; Nappi, E.; Quintina Muci, G.; Montagna, C.; Messina, M.R.; Ferri, S.; et al. Very Rapid Improvement of Extended Nitric Oxide Parameters, Associated with Clinical and Functional Betterment, in Patients with Chronic Rhinosinusitis with Nasal Polyps (CRSwNP) Treated with Dupilumab. J. Investig. Allergol. Clin. Immunol. 2022, 33, 1–25.
- Jeruzal-Świątecka, J.; Borkowska, E.; Łaszczych, M.; Nowicka, Z.; Pietruszewska, W. TAS2R38 Bitter Taste Receptor Expression in Chronic Rhinosinusitis with Nasal Polyps: New Data on Polypoid Tissue. Int. J. Mol. Sci. 2022, 23, 7345.
- Cantone, E.; Negri, R.; Roscetto, E.; Grassia, R.; Catania, M.R.; Capasso, P.; Maffei, M.; Soriano, A.A.; Leone, C.A.; Iengo, M.; et al. In Vivo Biofilm Formation, Gram-Negative Infections and TAS2R38 Polymorphisms in CRSw NP Patients. Laryngoscope 2018, 128, E339–E345.
- Carey, R.M.; Hariri, B.M.; Adappa, N.D.; Palmer, J.N.; Lee, R.J. HSP90 Modulates T2R Bitter Taste Receptor Nitric Oxide Production and Innate Immune Responses in Human Airway Epithelial Cells and Macrophages. Cells 2022, 11, 1478.
- Workman, A.D.; Maina, I.W.; Brooks, S.G.; Kohanski, M.A.; Cowart, B.J.; Mansfield, C.; Kennedy, D.W.; Palmer, J.N.; Adappa, N.D.; Reed, D.R.; et al. The Role of Quinine-Responsive Taste Receptor Family 2 in Airway Immune Defense and Chronic Rhinosinusitis. Front. Immunol. 2018, 9, 624.
- Qu, J.; Mei, Q.; Niu, R. Oxidative CaMKII as a Potential Target for Inflammatory Disease (Review). Mol. Med. Rep. 2019, 20, 863–870.
- Wang, H.; Do, D.C.; Liu, J.; Wang, B.; Qu, J.; Ke, X.; Luo, X.; Tang, H.M.; Tang, H.L.; Hu, C.; et al. Functional Role of Kynurenine and Aryl Hydrocarbon Receptor Axis in Chronic Rhinosinusitis with Nasal Polyps. J. Allergy Clin. Immunol. 2018, 141, 586–600.
- Esen, E.; Selçuk, A.; Passali, D. Epidemiology of Nasal Polyposis. In All Around the Nose; Cingi, C., Bayar Muluk, N., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 367–371. ISBN 978-3-030-21217-9.
- Rajguru, R. Nasal Polyposis: Current Trends. Indian J. Otolaryngol. Head Neck Surg. 2014, 66, 16–21.
- Traina, G.; Bolzacchini, E.; Bonini, M.; Contini, D.; Mantecca, P.; Caimmi, S.M.E.; Licari, A. Role of Air Pollutants Mediated Oxidative Stress in Respiratory Diseases. Pediatr. Allergy Immunol. 2022, 33 (Suppl. S27), 38–40.
- Istratenco, A. Oxidative Stress-Related Pathophysiology in Chronic Rhinosinusitis with Nasal Polyps: Research Challenges. Rom. J. Rhinol. 2019, 9, 71–77.
- Cho, D.-Y.; Le, W.; Bravo, D.T.; Hwang, P.H.; Illek, B.; Fischer, H.; Nayak, J. V Air Pollutants Cause Release of Hydrogen Peroxide and Interleukin-8 in a Human Primary Nasal Tissue Culture Model. Int. Forum Allergy Rhinol. 2014, 4, 966–971.
- Holecek, V.; Rokyta, R.; Slipka, J. Free Radicals in Nasal and Paranasal Diseases. In Free Radicals in ENT Pathology; Miller, J., Le Prell, C.G., Rybak, L., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 479–492. ISBN 978-3-319-13473-4.
- Topal, O.; Kulaksızoglu, S.; Erbek, S.S. Oxidative Stress and Nasal Polyposis: Does It Affect the Severity of the Disease? Am. J. Rhinol. Allergy 2014, 28, e1–e4.
- Okur, E.; Gul, A.; Kilinc, M.; Kilic, M.A.; Yildirim, I.; Tolun, F.I.; Atli, Y. Trace Elements in Nasal Polyps. Eur. Arch. Oto-Rhino-Laryngol. 2013, 270, 2245–2248.
- Cheng, Y.-K.; Hwang, G.-Y.; Lin, C.-D.; Tsai, M.-H.; Tsai, S.-W.; Chang, W.-C. Altered Expression Profile of Superoxide Dismutase Isoforms in Nasal Polyps from Nonallergic Patients. Laryngoscope 2006, 116, 417–422.
- Cekin, E.; Ipcioglu, O.M.; Erkul, B.E.; Kapucu, B.; Ozcan, O.; Cincik, H.; Gungor, A. The Association of Oxidative Stress and Nasal Polyposis. J. Int. Med. Res. 2009, 37, 325–330.
- Sagit, M.; Erdamar, H.; Saka, C.; Yalcin, S.; Akin, I. Effect of Antioxidants on the Clinical Outcome of Patients with Nasal Polyposis. J. Laryngol. Otol. 2011, 125, 811–815.
- Simsek, F.; Eren, E.; Bahceci, S.; Aladag, I. High PI3K/MTOR and Low MAPK/JNK Activity Results in Decreased Apoptosis and Autophagy in Nasal Polyposis. Braz. J. Otorhinolaryngol. 2021, 87, 572–577.
- Sahiner, U.M.; Birben, E.; Erzurum, S.; Sackesen, C.; Kalayci, Ö. Oxidative Stress in Asthma: Part of the Puzzle. Pediatr. Allergy Immunol. 2018, 29, 789–800.
- Okeleji, L.O.; Ajayi, A.F.; Adebayo-Gege, G.; Aremu, V.O.; Adebayo, O.I.; Adebayo, E.T. Epidemiologic Evidence Linking Oxidative Stress and Pulmonary Function in Healthy Populations. Chronic Dis. Transl. Med. 2021, 7, 88–99.
- Ricciardolo, F.L.M.; Bertolini, F.; Carriero, V.; Sprio, A.E. Asthma Phenotypes and Endotypes. Minerva Med. 2021, 112, 547–563.
- Kleniewska, P.; Pawliczak, R. The Participation of Oxidative Stress in the Pathogenesis of Bronchial Asthma. Biomed. Pharmacother. 2017, 94, 100–108.
- Kuruvilla, M.E.; Lee, F.E.-H.; Lee, G.B. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin. Rev. Allergy Immunol. 2019, 56, 219–233.
- Gans, M.D.; Gavrilova, T. Understanding the Immunology of Asthma: Pathophysiology, Biomarkers, and Treatments for Asthma Endotypes. Paediatr. Respir. Rev. 2020, 36, 118–127.
- Jones, T.L.; Neville, D.M.; Chauhan, A.J. Diagnosis and Treatment of Severe Asthma: A Phenotype-Based Approach. Clin. Med. 2018, 18, s36–s40.
- Katial, R.K.; Bensch, G.W.; Busse, W.W.; Chipps, B.E.; Denson, J.L.; Gerber, A.N.; Jacobs, J.S.; Kraft, M.; Martin, R.J.; Nair, P.; et al. Changing Paradigms in the Treatment of Severe Asthma: The Role of Biologic Therapies. J. Allergy Clin. Immunol. Pract. 2017, 5, S1–S14.
- Chamitava, L.; Cazzoletti, L.; Ferrari, M.; Garcia-Larsen, V.; Jalil, A.; Degan, P.; Fois, A.G.; Zinellu, E.; Fois, S.S.; Fratta Pasini, A.M.; et al. Biomarkers of Oxidative Stress and Inflammation in Chronic Airway Diseases. Int. J. Mol. Sci. 2020, 21, 4339.
- Allam, V.S.R.R.; Paudel, K.R.; Gupta, G.; Singh, S.K.; Vishwas, S.; Gulati, M.; Gupta, S.; Chaitanya, M.V.N.L.; Jha, N.K.; Gupta, P.K.; et al. Nutraceuticals and Mitochondrial Oxidative Stress: Bridging the Gap in the Management of Bronchial Asthma. Environ. Sci. Pollut. Res. 2022, 29, 62733–62754.
- Liu, K.; Hua, S.; Song, L. PM2.5 Exposure and Asthma Development: The Key Role of Oxidative Stress. Oxid. Med. Cell. Longev. 2022, 2022, 3618806.
- Tiotiu, A.I.; Novakova, P.; Nedeva, D.; Chong-Neto, H.J.; Novakova, S.; Steiropoulos, P.; Kowal, K. Impact of Air Pollution on Asthma Outcomes. Int. J. Environ. Res. Public Health 2020, 17, 6212.
- Jesenak, M.; Zelieskova, M.; Babusikova, E. Oxidative Stress and Bronchial Asthma in Children—Causes or Consequences? Front. Pediatr. 2017, 5, 162.
- Xu, C.; Song, Y.; Wang, Z.; Jiang, J.; Piao, Y.; Li, L.; Jin, S.; Li, L.; Zhu, L.; Yan, G. Pterostilbene Suppresses Oxidative Stress and Allergic Airway Inflammation through AMPK/Sirt1 and Nrf2/HO-1 Pathways. Immunity, Inflamm. Dis. 2021, 9, 1406–1417.
- Yong, J.I.; Kim, D.W.; Shin, M.J.; Jo, H.S.; Park, J.H.; Cho, S.B.; Lee, C.H.; Yeo, H.J.; Yeo, E.J.; Choi, Y.J.; et al. PEP-1-PEA15 Suppresses Inflammatory Responses by Regulation of MAPK in Macrophages and Animal Models. Immunobiology 2018, 223, 709–717.
- Zhang, X.; Ding, M.; Zhu, P.; Huang, H.; Zhuang, Q.; Shen, J.; Cai, Y.; Zhao, M.; He, Q. New Insights into the Nrf-2/HO-1 Signaling Axis and Its Application in Pediatric Respiratory Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 3214196.
- Li, J.; Wang, H.; Zheng, Z.; Luo, L.; Wang, P.; Liu, K.; Namani, A.; Jiang, Z.; Wang, X.J.; Tang, X. Mkp-1 Cross-Talks with Nrf2/Ho-1 Pathway Protecting against Intestinal Inflammation. Free Radic. Biol. Med. 2018, 124, 541–549.
- Wang, C.; Choi, Y.H.; Xian, Z.; Zheng, M.; Piao, H.; Yan, G. Aloperine Suppresses Allergic Airway Inflammation through NF-ΚB, MAPK, and Nrf2/HO-1 Signaling Pathways in Mice. Int. Immunopharmacol. 2018, 65, 571–579.
- Gu, X.; Zhang, Q.; Du, Q.; Shen, H.; Zhu, Z. Pinocembrin Attenuates Allergic Airway Inflammation via Inhibition of NF-ΚB Pathway in Mice. Int. Immunopharmacol. 2017, 53, 90–95.
- Yi, L.; Cui, J.; Wang, W.; Tang, W.; Teng, F.; Zhu, X.; Qin, J.; Wuniqiemu, T.; Sun, J.; Wei, Y.; et al. Formononetin Attenuates Airway Inflammation and Oxidative Stress in Murine Allergic Asthma. Front. Pharmacol. 2020, 11, 533841.
- Lim, J.-O.; Song, K.H.; Lee, I.S.; Lee, S.-J.; Kim, W.-I.; Pak, S.-W.; Shin, I.-S.; Kim, T. Cimicifugae Rhizoma Extract Attenuates Oxidative Stress and Airway Inflammation via the Upregulation of Nrf2/HO-1/NQO1 and Downregulation of NF-ΚB Phosphorylation in Ovalbumin-Induced Asthma. Antioxidants 2021, 10, 1626.
- Suzuki, M.; Cole, J.J.; Konno, S.; Makita, H.; Kimura, H.; Nishimura, M.; Maciewicz, R.A. Large-Scale Plasma Proteomics Can Reveal Distinct Endotypes in Chronic Obstructive Pulmonary Disease and Severe Asthma. Clin. Transl. Allergy 2021, 11, e12091.
- Saunders, R.M.; Biddle, M.; Amrani, Y.; Brightling, C.E. Stressed out—The Role of Oxidative Stress in Airway Smooth Muscle Dysfunction in Asthma and COPD. Free Radic. Biol. Med. 2022, 185, 97–119.
- Carpagnano, G.E.; Scioscia, G.; Lacedonia, D.; Soccio, P.; Quarato, C.M.I.; Cotugno, G.; Palumbo, M.G.; Foschino Barbaro, M.P. Searching for Inflammatory and Oxidative Stress Markers Capable of Clustering Severe Asthma. Arch. Bronconeumol. 2021, 57, 338–344.
- Lewis, B.W.; Ford, M.L.; Rogers, L.K.; Britt, R.D. Oxidative Stress Promotes Corticosteroid Insensitivity in Asthma and COPD. Antioxidants 2021, 10, 1335.
- Enweasor, C.; Flayer, C.H.; Haczku, A. Ozone-Induced Oxidative Stress, Neutrophilic Airway Inflammation, and Glucocorticoid Resistance in Asthma. Front. Immunol. 2021, 12, 631092.
