You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Ghassan Bkaily and Version 2 by Catherine Yang.
The vascular endothelium plays a vital role during embryogenesis and aging and is a cell monolayer that lines the blood vessels. The immune system recognizes the endothelium as its own. Therefore, an abnormality of the endothelium exposes the tissues to the immune system and provokes inflammation and vascular diseases such as atherosclerosis. Its secretory role allows it to release vasoconstrictors and vasorelaxants as well as cardio-modulatory factors that maintain the proper functioning of the circulatory system. The sealing of the monolayer provided by adhesion molecules plays an important role in cardiovascular physiology and pathology.
- endothelium
- endothelium physiology
- endothelium pathology
- endothelium dysfunction
2. The Vascular Endothelium
The vascular endothelium is a simple tissue in its morphology but complex in its function. Although it is formed as a single monolayer, it is capable of sensing hemodynamic and rheologic changes as well as responding to these modifications of its environment. Vascular tone is maintained by balancing vasodilating and vasoconstricting factors released by the endothelium. In addition, the endothelium plays a key role in controlling the migration and proliferation of VSMCs [1][2].
The integrity of the endothelial monolayer is essential to regulate vascular permeability and protect the vessel against platelet deposition and thrombus formation. Furthermore, the integrity of this monolayer requires that the morphology and the contacts between ECs do not change.
3. Origin and Differentiation of the Vascular Endothelium
The endothelium is the first cell type to constitute blood vessels. The formation of blood vessels and vasculogenesis result from the differentiation of the mesodermal cells into angioblasts due to the presence of specific proteins. These angioblasts are the precursors of endothelial and blood cells. The physiological primitive angiogenesis takes place to form the vascular tree, which gives birth to buds of the branches that give birth to the heart, including its endocardial endothelial cells. Then, remodeling of the vascular tree takes place to form capillaries and veins, including large arteries [1][2][3][2,10,11]. The endothelium of these newly formed blood vessels differs depending on the type of vessels. For example, fibroblast growth factor (FGF) receptors seem to be expressed only in large vessels [4][12]. Several ligands and their corresponding receptors are implicated in the differentiation and formation of the endothelium, including vascular endothelial growth factor (VEGF) and its receptors 1 and 2 [2][10].
The heart’s formation is more than a deformation of blood vessels, and differentiation of vascular endothelium into endocardial endothelium forms the left (arterial) and right (venous) endocardial endothelium layers. Both endocardial and vascular endothelium are separated from their muscle cells by a basal lamina membrane [1][2]. Vasculogenesis occurs during early embryonic development, whereas angiogenesis happens during adulthood. Angiogenesis is usually associated with diseases [3][11]. Several endothelial markers exist, such as VE-cadherin, PECAM-1, Tie-1 and 2, and flk1. Notch family activation plays an essential role in defining the characteristics and identities of arterial endothelial cells [5][13]. Although the molecular aspect of the arterial specification is more precise, little is known concerning the venous specification [5][13]. For example, the vascular endothelium can adapt its function depending on the environment. Still, it does not change its phenotype, such as in transplanted arterial and venous vessel grafts, where the graft vessel’s endothelium matches the host vessels’ characteristics [6][7][14,15].
4. Role of the Endothelium in Vascular Physiology
All blood vessels contain endothelial cells that form the intima. Two types of blood vessels only have endothelial cells: capillaries and venules. The intima is formed by continuous and discontinuous (fenestrated) layers of endothelial cells. Examples of continuous endothelium are arteries and veins. Tight, adherent junctions connect the continuous layer of endothelium side by side. Transport molecules go through this sealed monolayer of the endothelium via a transcytosis mechanism, such as caveolae (caveolin-1) and vesiculo-vacuolar organelles [8][16]. A fenestrated, discontinuous layer of endothelium permits extensive transport of molecules toward tissues such as the liver.
Several physiological functions are attributed to vascular endothelium, independent of their localization in the vascular tree, such as tuning the level of vascular endothelial cells (VECs) vasoconstrictors and vasorelaxers [1][9][2,17], regulation of coagulation and inflammation [10][11][3,18], and playing an essential role as a gatekeeper of fatty acids transport [12][13][14][15][16][17][19,20,21,22,23,24].
Among the vasoconstrictive factors released by the vascular endothelium are endothelin-1 (ET-1), thromboxane A2 (TxA2), as well as angiotensin-II (AngII) [1][8][9][18][19][2,16,17,25,26]. On the other hand, the most vasorelaxant factors released by the endothelium are nitric oxide (NO), prostacyclin (PGI2), and endothelium-derived hyperpolarizing factor (EDHF) [1][20][2,27].
The blood vascular system consists of a circuit of vessels in which the continuous movement of the heart pump maintains the blood flow. Blood vessels distribute nutrients, oxygen, and hormones to all organs and tissues and transport the products of cellular metabolism. The walls of arteries and veins, such as the thoracic aorta, used commonly in the literature, consist of three concentric tunics that are firmly joined from the inside out [1][2] (Figure 1): (1) The intima is the thin innermost layer that lines the various vascular walls, including those of the capillaries and venules. It is composed of a monolayer of endothelial cells (ECs) in direct contact with the blood and forming the vascular endothelium. The ECs provide a smooth inner surface that minimizes friction, which facilitates blood flow. The vascular endothelium is supported by a basal lamina and a thin connective tissue formed by collagen and some elastic fibers;
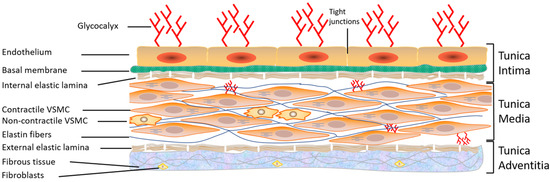
(2) The media is the thickest intermediate layer of the vascular wall. It consists of vascular smooth muscle cells (VSMCs), collagen, and elastin. This layer is absent in the capillaries and venules; (3) The adventitia is the outermost layer of the vascular wall. It is absent in capillaries and venules. This layer is formed of supporting connective tissue consisting mainly of collagen. It is also crossed by numerous nerve endings controlling the activity of the muscle fibers as well as the blood vessels feeding the vascular wall, called vasa vasorum (vessels of the vessels). The relative importance of these three layers varies according to the type of blood vessel [1][2]. In conclusion, all blood vessels have an endothelium but not necessarily adventitia or VSMCs, hence the importance of studying and learning more about the vascular endothelium.
5. Structure of the Vascular Endothelium of Arteries and Veins
A monolayer of flat cells forms the vascular endothelium of arteries and veins, with a central nucleus measuring 10–20 µm in diameter. VECs are characterized by extensive intercellular overlap and long, deep slits that contribute to the integrity of the vascular endothelium [1][2]. The integrity of this monolayer is ensured by a dynamic cytoskeleton [1][21][22][23][2,28,29,30] as well as by contacts between cells and between these cells and the extracellular matrix [1][24][25][2,31,32]. In vivo and in situ morphology studies have shown the presence of tight junctions, adhesion junctions, and gap junctions between adjacent VECs (including aortic VECs) [25][26][27][32,33,34]. In addition, several roles have been attributed to junctional communication at the vascular endothelium level, including intercellular nutrient exchange, regulation of growth and differentiation, coordination of cellular response to exogenous and endogenous stimuli, and maintenance of vascular tissue homeostasis [28][29][30][31][35,36,37,38]. The cytoskeleton is well-developed in ECs. It contributes to vascular homeostasis and seems to play an essential role in the repair and integrity of these cells [21][22][23][28,29,30]. VECs contain the actin protein in its filamentous polymeric form, called F-actin, and in its globular monomeric form, called G-actin [32][33][39,40]. Therefore, F- and G-actin play a role in the shape of ECs. The balance between the monomeric and polymeric forms could be altered during stimulation of ECs and contribute to the modulation of intercellular junctions that affect the vascular permeability of the endothelial layer. Indeed, during the migration of VECs, G-actins increase compared to F-actins [34][41]. The migration of these cells also involves the redistribution of centrosomes [21][28]. Actin microfilaments are localized within the cell as short, thin stress fibers and form a continuous band at the periphery [21][32][28,39]. In situ studies have also demonstrated the presence of the protein myosin at the level of these microfilaments [35][36][42,43], which plays an essential role in cell adhesion, and facilitates the adaptation of the vascular wall to variations in blood flow pressure [21][28]. The presence or absence of an actin isoform allows the identification of ECs. Therefore, the presence of α-actin in VECs is considered to be a marker for this type of cell [37][44].6. Role of the Endothelium in Vascular Activity
ECs respond to chemical and physical stimuli by synthesizing and releasing various vasoactive and growth factors [1][2] (Figure 2). The endothelium possesses anti-adhesive substances that prevent blood from clotting. The anticoagulant and antithrombotic properties of the vascular endothelium, which are essential for vascular homeostasis, are due to the synthesis of vasodilatory factors such as nitric oxide (NO) and prostacyclin [8][35][38][39][40][5,16,42,45,46] (Figure 2). On the other hand, the vascular endothelium secretes several vasoconstrictor substances (Figure 2), including endothelin-1 (ET-1), prostaglandins, and several components of the renin-angiotensin system (RAS), such as angiotensin II (Ang II). Ang II [18][19][41][42][25,26,47,48] and ET-1 [43][44][45][49,50,51] act at the plasma and nuclear membranes of ECs and induce an increase in the intracellular calcium level via activation of their respective receptors, AT1/AT2 and ETA /ETB receptors. This increase in [Ca2+]i may, in turn, modulate the secretory function of ECs [1][46][2,52] and survival [19][44][26,50]. Furthermore, a balance between the different factors secreted by the EV is essential for maintaining intracellular homeostasis and wall integrity. Any disturbance in this balance leads to endothelial dysfunction, characterized by a decreased capacity for relaxation of the vessel, an increase in the adhesion of blood cells to the vascular wall, and a disturbance in the tunica medial [13][38][47][48][49][50][51][52][1,5,9,20,53,54,55,56]. This endothelial dysfunction is generally observed during aging and in several vascular pathologies, such as hypertension, hypotension, atherosclerosis, and heart failure [19][53][54][26,57,58]. All VECs synthesize and secrete von Willebrand factor (vWF), a multifunctional protein involved in the typical arrest of hemorrhage [55][59]. Indeed, through its interaction with extracellular matrix proteins and membrane receptors, vWF plays a prominent role in blood coagulation, platelet aggregation, and platelet adhesion to the extracellular matrix [56][57][60,61]. vWF can also bind to the pro-coagulant co-enzyme, factor VIII, contributing to its stability and, indirectly, to the production of fibrin [56][57][60,61]. vWF is stored in small vesicles characteristic of endothelial cells, the Weibel–Palade bodies [56][57][58][60,61,62]. The latter contain other proteins, such as ET-1 [58][59][62,63] and interleukin-8 [60][64]. In addition, vWF is used as a marker of ECs in vitro [61][65].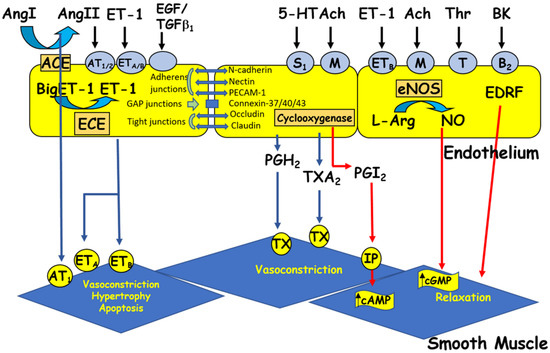
Figure 2. The endothelium produces vasoactive factors that cause either relaxation or contraction of the vascular smooth muscle. Ang I and II: angiotensin I and II, ACE: angiotensin-converting enzyme, Ach: acetylcholine, BK: bradykinin, cAMP/cGMP: cyclic adenosine/guanosine monophosphate, ECE: endothelin-converting enzyme, EDRF: endothelium-derived relaxing factor, ET-1: endothelin-1, 5HT: 5-hydroxytryptamine (serotonin), L-Arg: L-arginine, NO: nitric oxide, NOS: nitric oxide synthase, PGH2: prostaglandin H2, PGI2: prostacyclin, TGFβ1: transforming growth factor beta 1, Thr: thrombin, and TXA2: thromboxane A2. Circles represent receptors (AT: angiotensin receptor, B: bradykinin receptor, ET: endothelin receptor, M: muscarinic receptor, IP: purinergic receptor, S: serotonin receptor, T: thrombin receptor, and TX: thromboxane receptor).
