The human body harbors trillions of microbes of different kinds performing various physiological activities, such as priming the immune system, influencing host metabolism, and improving health by providing important metabolites such as short-chain fatty acids. Although the gut is considered the “microbial organ” of our body as it hosts the most microbes, there are microbes present in various other important anatomical locations differing in numbers and type. Research has shown the presence of microbes in utero, sparking a debate on the “sterile womb” concept, and there is much scope for more work in this area. It is important to understand the early-life microbiome colonization, which has a role in the developmental origins of health and disease in later life.
1. Introduction
While there are still inconsistencies about who was the first to coin the terms microbiota and microbiome, it is accepted that “microbiota” is a collection of microorganisms in a defined environment. The most accepted definition of the term “microbiome” is the collection of all the microbial genes or genomes of the microbiota
[1]. Others in the field extend the microbiome definition to the entire habitat, including bacteria, viruses, fungi, archaea, and others
[2]. Moreover, a few utilize the term “metagenome”: a collection of genes and genomes of the microbiota, which comes from molecular methods such as “metagenomics”
[1][2][1,2]. Metagenomics methods, such as 16S rRNA or Whole Genome Sequencing (WGS), are the next-generation sequencing methods to get microbial information from a given biological sample
[3]. Moreover, this sequencing allowed the sampling of the principal multi-kingdom species present within a microbiome. Each sequence is assigned to individual microbial taxon such as bacteria, viruses, fungi, and archaea from phylum to species taxonomic levels. The novel terms with respect to an individual microbial taxon that are emerging in microbiome research include “bacteriome” (bacterial communities), “virome” (viral communities) including phageome (phage communities), “archaeome” (archaeal communities), and “mycobiome” (fungal communities)
[3]. All these multi-kingdom microbial communities interact with each other and play an important role throughout the human lifecycle, even before birth. The long-standing sterile womb hypothesis is challenged by emerging research, altering previous understanding of microbiome establishment after birth
[4][5][6][4,5,6]. These studies have identified microbes and their products in amniotic fluid, placenta, and meconium, demonstrating the in utero microbial establishment, which continues during the first three years of life. However, the sources of these in-utero microbes and the routes by which they access the fetal gut remain largely unknown. A few studies have indicated that these microbes are maternally sourced mainly due to bacteria found in cord blood, however, the root can go back to genital microbiomes
[7][8][9][7,8,9]. Perturbations in these different biomes during prenatal to neonatal periods can have short- and long-term consequences in an infant’s life, such as preterm birth, autoimmune disorders, chronic lung disease, inflammatory bowel diseases, eczema, asthma, obesity, and central nervous system disorders.
The following sections review novel areas of microbiome research beyond the gut, along with a few new “biotas” and “biomes” for the very first time (Figure 1), and provide a novel perspective on developmental origins of the microbiome and concisely discuss the importance of research beyond the gut microbiome.
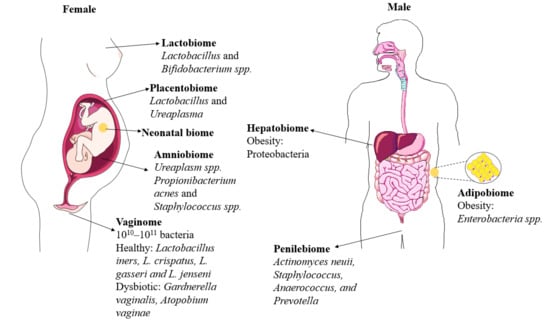
Figure 1. Overview of emerging biomes. Beyond the gut, emerging microbiomes in human body sites (in both males and females) influence overall health and well-being. This figure provides information about the bacterial microbiome.
2. Genital Microbiomes
2.1. Vaginobiome
The terms vaginobiota and vaginobiome (or vaginome), for very first-time use, describe the collection of microbial communities and their genome of the vaginobiota in the female reproductive tract. Vaginobiome is a dynamic micro-ecosystem, which is constantly influenced by the menstrual cycle and immune system, and this bi-directional crosstalk is known as microgenderome
[10][11][12][10,11,12]. In healthy females,
Lactobacillus spp. is dominant in the vaginobiome
[13] (
Table 1). A variety of microbes are transferred from male to female partners during copulation and dysbiosis in the vaginobiome, which can be linked to intrauterine microbial sources
[14]. Understanding the genital microbiomes can lead to understanding sexually transmitted infections or related health outcomes and research into prevention.
Table 1.
Overview of microbiome clinical studies other than gut discussed herein.
2.2. Penilebiome
The terms penilebiota and penilebiome, for very first-time use, describe the collection of microbial communities and their genome of the penilebiota in the male reproductive tract.
Actinomyces neuii,
Staphylococcus, Anaerococcus, and
Prevotella are the genera that are more abundant over time
[14][16][14,16].
3. Microbiomes Associated with the Early Life Development
3.1. Amniobiome
The terms amniobiota and amniobiome, for very first-time use, describe the collection of microbial communities and their genomes of amniobiota in amniotic fluid. A multitude of studies suggests the presence of microbes in utero employing advanced next-generation sequencing technologies, which is a paradigm shift in the idea of a sterile intrauterine environment
[5][7][8][5,7,8]. Specifically,
Ureaplasma spp. was detected in the amniotic samples at 16–20 weeks gestation and post-third trimester
[17]. Propionibacterium acnes and Staphylococcus spp. are the few other species reported in amniotic fluid. In addition, bacterial communities were identified in the meconium and the placenta concluding that the microbial colonization of the fetal gut begins in utero and continues during the first two years of life
[5][18][5,18]. Indeed, alteration of the placental and amniotic fluid bacteriome has been associated with preterm birth
[26].
3.2. Placentalbiome
The collection of microbes in the placenta can be termed placentalbiota, and their genomes together are termed placentalbiome. The placenta is an exchange site of nutrients and blood between the fetal and maternal systems and performs vital metabolic functions supporting fetal development and maintaining maternal-fetal tolerance
[27]. Interestingly,
Lactobacillus and
Ureaplasma spp. were most commonly found in the placenta
[19][28][19,28], where
Lactobacillus spp. was associated with the healthy human gut, breast milk, and vaginome.
3.3. Meconiobiome
The collection of microbes in meconium can be termed meconiobiota, and their genomes together are termed meconiobiome or meconiome. Meconium is the first postnatal bowel movement of infants. The post-birth meconium microbiome is thought to represent the in-utero microbial environment
[18]. Regardless of whether meconium is colonized before, during, or after birth, the composition and structure of early-life gut communities may influence health later in life. Meconium mostly shared dominated by
Lactobacillus,
Bifidobacterium, Staphylococcus, and
Enterococcus spp.
[15].
3.4. Lactobiome
Besides the mode of delivery and environment, early nutrition through breastfeeding is a key factor directing the neonatal microbiota composition. The collection of microbes in breast milk can be termed lactobiota, and their genomes together are termed lactobiome. Breastmilk is considered a seed microbiome in an infant’s gut, and the components of milk nurture the microbiome with beneficial bacteria. Lactobiome is mostly dominated by beneficial bacteria such as
Lactobacillus and
Bifidobacterium spp.
[20][21][20,21]. Beneficial gut microbes play a role in lowering the risk of later-life chronic diseases like asthma, obesity, allergies, dermatitis, inflammatory bowel disease, and neurodevelopmental disorders
[29].
4. Microbiomes Associated with Later-Life Metabolic Health: Emerging Research Areas
For a long period, metabolic tissues such as adipose and liver tissues were considered sterile sites and, as such, were not examined for the presence of the microbiome. However, emerging studies have clearly demonstrated the presence of microbes in metabolic tissues.
4.1. Adipobiome
Recently, a few studies have identified bacterial signatures in metabolic tissues, such as adipose tissue and the liver. The collection of microbes in the adipose tissue can be termed adipobiota, and their genomes together are termed adipobiome. Human studies demonstrated an association of certain Proteobacteria phylum members, such as
Enterobacteria spp., to the initiation of subclinical inflammation, influencing metabolic pathways leading to obesity
[22][23][22,23]. Although the physiological role and source of these bacteria are still under debate, microbial translocation is identified as the viable route. In addition, early-life exposure to microbes in the womb might be an interesting hypothesis for the microbial source in the metabolic tissues
[22][30][22,30].
4.2. Hepatobiome
Similar to the adipobiome, the collection of microbiota in the liver can be termed hepatobiota, and their respective genome is termed heptabiome. Suppli et al. and a few other researchers examined liver biopsies from lean and obese individuals. They found that the composition of bacterial DNA in the livers of the obese group differed from the lean group
[24][25][31][24,25,31]. In addition, heptabiome followed similar patterns of higher Proteobacteria phylum in the obese group, suggesting a liver-adipose axis in the development of the metabolic syndrome. Leinwand et al. also found the presence of heptabiome in mice and humans, where higher abundances of Proteobacteria induced inflammation
[25]. These findings provided a rationale for microbiome-based therapies in treating liver disease.