Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Maria Yu Zakharova and Version 3 by Conner Chen.
Antigen presentation by major histocompatibility complex class II (MHC-II) molecules is crucial for eliciting an efficient immune response by CD4+ T cells and maintaining self-antigen tolerance. Some MHC-II alleles are known to be positively or negatively associated with the risk of the development of different autoimmune diseases (ADs), including those characterized by the emergence of autoreactive T cells. Apparently, the MHC-II presentation of self-antigens contributes to the autoimmune T cell response, initiated through a breakdown of central tolerance to self-antigens in the thymus.
- autoimmune diseases
- autoreactive T cells
- human leukocyte antigen
1. MHC-II Presentation of Low-Affinity Self-Antigens
Major histocompatibility complex class II (MHC-II) molecules are synthesized in the endoplasmic reticulum (ER); the peptide-binding groove is occupied by the invariant chain Ii (CD74 fragment) to prevent degradation and premature binding of self-antigens by the complex. As they migrate to the late endosomal compartments with an acidic environment, proteolytic enzymes shorten the invariant chain to a shorter class II-associated invariant chain peptide (CLIP) [1][28]. Protein antigens are proteolytically processed in endosomes as well (Figure 12). The peptide-binding groove of an MHC class II molecule has nine pockets that can accommodate certain amino acid residues of the peptide, typically stabilized by noncovalent bonds [2][29]. The P1, P4, P6, and P9 anchor residues interact with the groove of MHC-II, thus forming a binding register, while the remaining residues of the peptide are oriented in the opposite direction for T cell receptor (TCR) binding. Presumably, if there is a shift in the binding register between antigenic peptides and MHC molecules, the peptide–MHC-IIMHC complex (pMHC) complex ccan interact with an entirely different TCR.
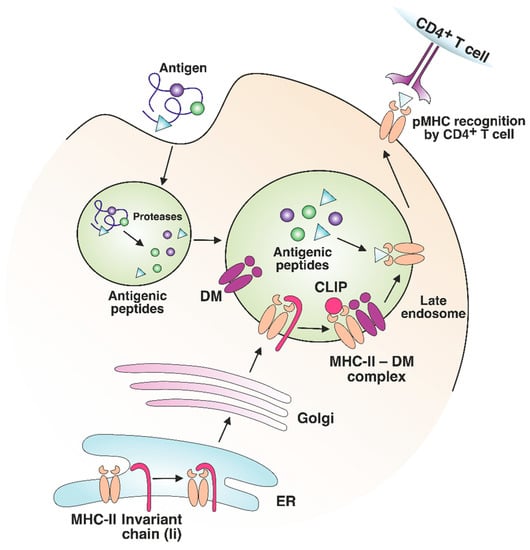
Figure 12. MHC-II maturation and antigenic peptide loading. MHC-II molecules are synthesized in the endoplasmic reticulum (ER) and loaded with an invariant chain (Ii). The MHC-II complex with Ii is transported through the Golgi to the late endosome. Endosomal proteases process antigens to short peptides and Ii to shorter class II-associated invariant chain peptide (CLIP). The binding of a nonclassical HLA-DM (DM) molecule to MHC-II promotes CLIP exchange to the antigenic peptide with an optimal binding register. The formed pMHC complex is transported to the surface of the antigen-presenting cell (APC) for CD4+ T cell recognition.
The nonclassical MHC molecules HLA-DM (DM) and HLA-DO (DO) in humans and H2-DM and H2-DO in mice play an important role in exchanging CLIP for endosomal antigens [3][30]. By interacting with MHC-II, DM has the “editing” function: it ensures the binding of peptides with higher affinity for the MHC molecule compared with CLIP, as well as increases the loading/dissociation rate of the antigen, but it causes no effect on the equilibrium affinity [4][5][31,32]. Apparently, DM facilitates the presentation of antigen epitopes with the optimal binding register on MHC-II [6][7][33,34]. DO also performs an editing action, interacting with DM and regulating its catalytic function by preventing its binding to MHC-II [8][35]. Comparing MHC-II immunopeptidomes from two cell lines, DO knockout or not, it was shown that only the DR1+, DM+, and DO+ lymphoblastoid cell lines presented specific antigens on MHC-II [9][36]. Thus, DO contributes to the diversification of the MHC-II-presented antigenic repertoire. In experiments with DM-knockout mice, CLIP was not efficiently exchanged with other peptides, thus limiting the MHC-II antigen diversity and causing incomplete negative selection of CD4+ T cells in the thymus [10][37]. The repertoire of antigens presented by MHC-II can vary significantly depending on the DM/DO ratio in the cell [11][38]. Presumably, DM “editing” decreases the number of antigens, characterized by a low affinity for MHC-II, on the antigen-presenting cell (APC) surface, as was shown for DQ1 and DQ6 [12][39], as well as for DR3 alleles [4][31]. Analysis of the immunopeptidome of thymic antigen-presenting cells (APCs) revealed that most antigens detected on MHC-II have a high affinity for MHC-II molecules [13][14][40,41]. Most likely, the effect of nonclassical MHC molecules is one of the important factors ensuring the highly competitive conditions for MHC-II ligand binding, which eventually results in the presentation of higher-affinity antigens.
It should be emphasized that the presentation of preferentially high-affinity antigens in the thymus may have its own shortcomings. It has been demonstrated that the MHC-II-presented antigen repertoire from tissues affected by autoimmune processes mostly consists of low-affinity peptides [15][16][42,43]. The abundance of self-antigens increases in peripheral tissues (e.g., myelin basic protein (MBP) in the central nervous system (CNS) [17][18][19][44,45,46] and insulin in the pancreas [20][47]). These protein self-antigens can be processed in the extracellular environment outside APCs. Next, the resulting antigenic fragments, characterized by a low affinity for MHC-II molecules, can be loaded directly on the APC surface or can enter early endosomes with low DM levels, thus escaping DM editing, and as a result, can be presented on the cell surface (Figure 23A,B). Certain MHC-II risk alleles bind CLIP with diminished affinity, which additionally promotes the binding of low-affinity antigenic peptides [21][22][48,49]. Rapid dissociation of CLIP enables the generation of empty complexes without DM involvement, which may potentially bind low-affinity antigenic peptides. Due to the spontaneous release of CLIP, low-affinity antigenic peptides are able to bind MHC-II in early endosomes without DM assistance or directly on the cell surface. Therefore, pMHC complexes on the APC surface at the periphery can activate T cell receptors (TCRs) that have not undergone negative selection due to the low abundance of this pMHC in the thymus. For multiple sclerosis (MS)MS patients, there was detected an immune response to full-length MS autoantigen proteolipid protein (PLP), naturally processed by APCs, with 2 immunodominant epitopes generation. Alternatively, the T cell response to several PLP synthetic fragments with high affinity to MS-associated MHC-II alleles was also observed after additional T cell stimulation by these peptides [23][50]. Since these fragments are normally hidden in a protein fold and are inaccessible for the endosomal proteases, the T cell activation seems to be the result of extracellular PLP processing and loading on MHC-II. Thus, the classical intracellular processing of antigen involves its capture by the APC with proteolytic processing and generation of fragments with optimal binding registers with the participation of HLA-DM in late endosomes, followed by subsequent exposure of pMHCs on the APC surface. These pMHCs take part in the negative selection process in the thymus, induce T cell clonal deletion and, after all, no autoreactive T cell response is observed at the periphery. Since pMHCs with “suboptimal” fragments were present in the thymus at low levels, autoreactive T cells might engage them at the periphery due to the failure of negative selection in the thymus.
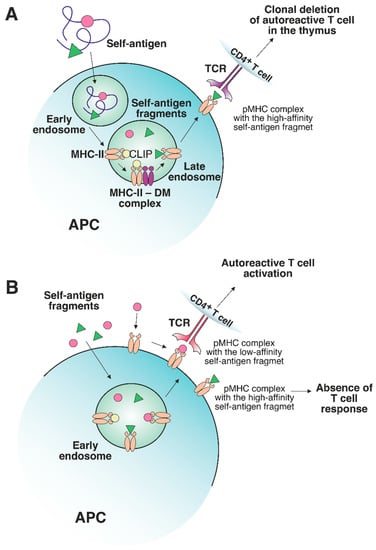
Figure 23. The role of a nonclassical DM molecule in the editing of the self-antigen repertoire presented by MHC-II. (A) In a thymic APC, the self-antigen is processed through a classical pathway under exposure to cellular proteolytic enzymes. Next, CLIP is exchanged to the high-affinity fragment in late endosomes under DM control. The stable pMHC complex is then transferred to the APC surface, where the T cells specific to this pMHC undergo clonal deletion. (B) If an excessive amount of certain self-antigens, processed outside APCs, is present in peripheral tissues, then these self-antigen fragments can be loaded on MHC-II molecules in early endosomes or on the surface of APCs without the recruitment of DM. The absence of DM editing allows the emergence of either stable or unstable pMHC complexes. The pMHCs, containing high-affinity fragments, do not elicit a T cell response due to the deletion of autoreactive T cells in the thymus. Unstable complexes with low-affinity fragments can bind autoreactive T cells since such T cells are not susceptible to clonal deletion.
2. The Peculiarities of the Interaction between TCRs and Self-pMHC Complexes: Links with Autoimmunity
Generally, CD4+ T cell TCRs recognize foreign antigens within MHC-II molecules [24][25][26][58,59,60] with CD4 receptors playing the key role in the induction of the T cell signaling cascade [27][28][29][61,62,63]. The analysis of trimolecular complexes between TCRs, MHC-II, and exogenous antigens has revealed some structural similarity in the TCR binding topology [30][64]. The TCR is arranged diagonally with respect to the antigen residing in the peptide-binding groove limited by α-helices. The variable regions of α and β TCR chains interact with the β and α chains of the MHC-II molecule, respectively [31][65]. The most structurally diverse CDR3 fragments of α and β TCR chains are located above the P5 residue of the bound peptide, while the CDR1 and CDR2 fragments interact with the α-helices of MHC-II molecules. The binding of TCR HA1.7 to the fragment of hemagglutinin HA protein within DR1 is an example of canonical interaction [32][66].
It was shown that, by contrast with TCRs binding bacterial or viral antigens on MHC-II, some autoreactive TCRs have an alternative binding topology. Thus, the TCR from an MS patient (Ob.1A12) specific for the fragment of myelin basic protein (MBP), which is presented on the HLA-DR2b risk allele molecule, interacts mainly with the N-terminus of the MBP fragment [33][16]. Furthermore, this TCR does not reside in the canonical diagonal position: its orientation angle is 110°, as opposed to 70° for HA1.7. The main interaction with the pMHC complex occurs due to the binding of the CDR3 region of TCR with the P2 residue of the MBP peptide fragment. Other TCRs, Ob.2F3 and Ob.3D1, obtained from the same donor with MS, also have an alternative binding topology, as examined using computational simulation [34][67]. Another TCR, 3A6, binds the MBP fragment in the complex with different DR15 allomorph DR2a, which is positively associated with the risk of developing MS. The 3A6 also has a suboptimal topology and low affinity for pMHC, similar to other autoreactive TCRs [35][68]. Its CDRs are shifted toward the N-terminus of the antigen, and CDR3 is also located above P2 of the MBP fragment. Nevertheless, similar to HA1.7, 3A6 binds diagonally with respect to pMHC. Therefore, Ob.1A12 and 3A6 have low affinity for pMHC, and their binding topology is alternate to anti-viral and anti-bacterial TCRs, which may potentially facilitate the escape from negative selection mechanisms in the thymus. Low-affinity TCRs were also reported in the pathogenesis of type 1 diabetes (T1D)T1D. The repertoire of the autoreactive T cells, infiltrating pancreatic islets, exhibited low self-reactivity and promoted the development of T1D [36][69]. Presumably, the interaction between these T1D TCRs and self-antigen-carrying pMHCs is also characterized by alternate topology. The MBP fragment presented by DQ1 is recognized by the Hy.1B11 TCR with relatively high affinity [37][70]. These TCRs have canonical localization but are significantly shifted with respect to the DQ1α chain so that their interaction with the antigen is substantially limited. Only the CDR3 region of the Hy.1B11α chain contacts the MBP fragment. Presumably, the unusual topology of autoreactive TCRs binding to pMHC impedes activation by the CD4 molecule and, therefore, clonal deletion of T cells at the negative selection stage. This may explain the existence of T cells with similar TCRs (such as Hy.1B11) in the periphery [38][71]. The unusual topology of the trimolecular complex often implies the participation of a low-affinity TCR. However, autoimmune TCRs with a high affinity for pMHCs have also been reported. It is known that DQ molecules have a lower expression level than DR molecules. Therefore, appropriate autoreactive TCRs might require higher affinities for DQ pMHC complexes to overcome clonal deletion in the thymus [37][70]. Another possible explanation of high-affinity autoreactive TCR emergence at the periphery is that the autoantigenic fragment may be a weak binder in the pMHC complex. As previously discussed, pMHC carrying low-affinity peptides allows T cells to escape negative selection. Nevertheless, they can further bind autoreactive TCRs at the periphery. Interestingly, the weak interaction between the MBP fragment and the product of the risk allele DR4 is stabilized by the MS2-3C8 TCR involved in canonical high-affinity interaction with pMHC [39][72]. The structures of the trimolecular complexes of three autoreactive TCRs in model mice with experimental autoimmune encephalomyelitis (EAE), which bind the MBP fragment in complex with I-Au, have revealed canonical binding.
3. The Effect of MHC-II-Presented Self-Antigen Modifications on Autoreactive TCR Recognition
Antigens processed for further presentation by MHC-II molecules can undergo various posttranslational modifications (PTMs), including glycosylation, iodination, citrullination, etc. (Figure 34). PTMs can either occur spontaneously or be induced by various enzymes. Autoreactive T cell responses to modified self-antigens have been observed for many autoimmune diseases (ADs). Supposedly, the modified antigens (neoantigens) are either totally not present in the thymus or their quantity is extremely low. These modifications presumably take place in peripheral tissues and are absent when the antigen is presented by medulla thymic epithelial cell (mTEC)TEC cells in the thymus, which has been demonstrated for glycosylated type II collagen for the development of rheumatoid arthritis (RA)RA [40][76]. Although modified antigens are available in the thymus due to the presence of migratory APCs, their quantity is probably insufficient for negative T cell selection in the thymus. Thus, thyroglobulin, which is the autoantigen in patients with Hashimoto’s thyroiditis, is present in the thymus in its nonmodified form, whereas the level of its iodinated form, which seems to initiate the autoreactive T cell response, is extremely low in the thymus and is insufficient for central tolerance to be established [41][77].
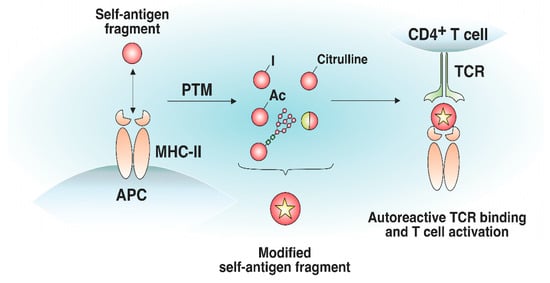
Figure 34. Effect of posttranslational modifications of self-antigens on trimolecular complex assembly. Certain self-antigen fragments do not bind to MHC-II molecules, or their binding affinity is low. Similar self-antigen with posttranslational modifications (iodination, acetylation, glycosylation, citrullination, generation of hybrid peptides, etc.) exhibiting increased affinity for MHC-II molecules, can appear in peripheral tissues. The resulting pMHC complexes can bind autoreactive T cells that have not undergone negative selection in the thymus.
Citrullination of antigens is described for a number of ADs, especially for RA; the positive charge of arginine is lost due to the conversion to citrulline, which ultimately alters the epitope affinity to MHC-II and TCR [42][78]. The products of risk MHC-II alleles for RA carry a consensus amino acid sequence in the antigen-binding groove, forming a positively charged P4 pocket–shared epitope (SE), which binds the polar amino acid residues of peptides at the P4 position (e.g., citrulline) and induces the activation of autoreactive T cells [43][44][79,80]. In addition to hosting a polar-neutral citrulline residue, SE can itself serve as the main contact zone for autoreactive TCRs and represent citrullinated fragments of the fibrinogen autoantigen that directly interact with autoreactive TCRs via the P2-citrulline residue [45][81]. Autoreactive T cells specific for citrullinated tenascin-C antigenic peptides, presented by DR4 molecules, were revealed in RA patients [46][82]. Additionally, glycosylation was reported to be responsible for the pathogenesis of RA. Analysis of the crystal structure of the trimolecular complex of an autoreactive TCR and type II collagen (Col2) neoantigen presented on DR4 has shown that lysine residues subjected to galactosylation are the key sites for TCR recognition [47][83]. Furthermore, the autoreactive T cell response is dependent on the galactosylation of lysine in the Col2259–273 fragment at position 264. Modified autoantigenic peptides also participate in T1D development. Several citrullinated and transglutaminated GAD65 epitopes are recognized by autoreactive CD4+ T cells in T1D preferentially to their unmodified form [48][84]. Autoreactive T cells specific for citrullinated glucokinase epitopes are linked to T1D pathogenesis [49][85]. Another example of the modified antigens impacting the pathogenesis of T1D is the generation of a disulfide bond. T cells recognize insulin fragments presented on DR4 molecules in T1D upon the assembly of a disulfide bridge between neighboring cysteine residues [50][86]. The citrullinated fragments of MBP and myelin oligodendrocyte glycoprotein (MOG) cause EAE due to the activation of autoreactive T cells [51][52][53][87,88,89]. In EAE, acetylation of the MBP fragment elicits an encephalitogenic T cell response [54][55][56][73,74,75].
4. The Effect of the Proinflammatory Environment on MHC-II Antigen Presentation and Autoreactive T Cell Engagement
The generation and expansion of pathological T cells may be attributed to the increase in MHC-II expression levels due to the proinflammatory environment induced by viral or bacterial infections. The impacts of viral and bacterial infections on antigen presentation are the proposed triggers of pathological CD4+ T cell expansion in ADs such as MS and T1D [57][58][59][60][61][98,99,100,101,102]. Earlier, a proinflammatory environment was implicated in TCR-independent bystander activation in different ADs [62][63][103,104]. Proinflammatory cytokine release and bacterial antigen presence trigger the elevated synthesis of MHC-II molecules to maximize the presentation of foreign antigens for an efficient CD4+ T cell response. High IFN-γ levels increase the number of MHC-II molecules on professional and nonprofessional APCs, thereby altering the composition of the MHC peptidome in the inflammatory area [64][105]. Additionally, APCs, activated in pathogenic conditions, express an increased number of costimulatory molecules [65][66][106,107]. Therefore, rare self-antigen pMHC, which cannot induce an autoreactive T cell response under normal conditions, might engage autoreactive TCRs in a proinflammatory environment (Figure 45). Concluding, cytokines induced by the inflammatory environment may potentially activate antigen-specific autoreactive T cells.
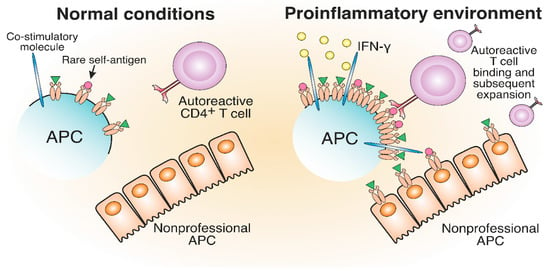
Figure 45. The development of autoreactive CD4+ T cells in a proinflammatory environment. The proinflammatory environment caused by viral or bacterial infection entails IFN-γ production, which results in increased MHC-II and costimulatory molecule expression compared to normal conditions. Therefore, rare self-antigens are present in a greater number, which can lead to the potential activation of autoreactive T cells.
A correlation between the inflammatory milieu and elevated MHC-II levels has been observed for several ADs. It was shown that MHC-II transcripts were upregulated by proinflammatory cytokine expression in β-cells of T1D patients [67][68][108,109]. IFN-γ induces the expression of I-Ag7 molecules on beta cells of non-obese diabetic (NOD) mice, leading to the emergence of CD4+ autoreactive T cells and the promotion of T1D [69][110]. In addition, IFN-γ promotes the expression of MHC-II on islet endothelial cells in NOD mice [70][111]. The transcription factors responsible for the expression of MHC-II are elevated in MS lesions of human brain tissue [71][112]. Aberrant MHC-II expression was also found on oligodendrocytes in EAE mice and MS patients [72][113]. The expression of MHC-II in mouse joints led to the development of severe erosive inflammatory polyarthritis [73][114]. Additionally, the inflammatory environment promotes the expression of MHC-II molecules on hepatocytes during autoimmune hepatitis and pMHC transfer via trogocytosis to interacting CD4+ T cells, which further amplifies the autoimmune response [74][115].
5. Molecular Mimicry between Self- and Foreign MHC-II Antigens May Lead to T Cell-Mediated Autoimmunity
Molecular mimicry refers to a structural similarity between self and exogenous antigens and potentially leads to autoimmunity [75][76][77][122,123,124]. The structural resemblance of antigens might result in the occasional misactivation of T cells (Figure 56). Autoreactive T cells from MS patients specific for MBP can cross-react with epstein–barr virus (EBV) nuclear antigen 1 (EBVNA1) [78][79][125,126]. Apparently, the virus-specific TCR was able to bind structurally similar self-antigen fragments presented on MHC-II and initiate an autoreactive T cell response. The probability of MS development is significantly increased when EBV infection is combined with the MHC-II risk allele HLA-DRB1*15:01. It was shown that CD4+ T cells specific for DR15-presented EBV antigens could cross-react with MBP [80][127].
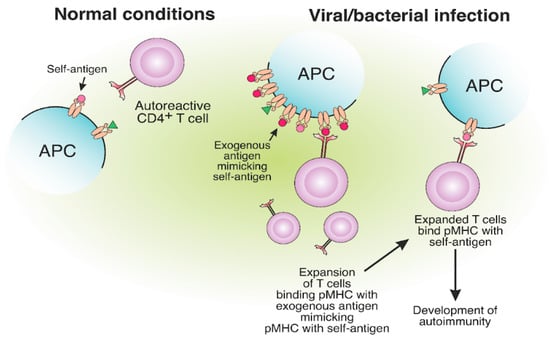
Figure 56. The expansion of autoreactive CD4+ T cells due to the molecular mimicry between foreign and self-antigens. The presentation of viral or bacterial antigenic peptides on MHC-II molecules, structurally similar to self-antigens, may result in the expansion of CD4+ T cells, specific to exogenous pMHC. Expanded CD4+ T cells, specific for foreign peptides, can bind the self-antigen pMHC, which results in the development of autoimmunity.
Narcolepsy is a rare autoimmune chronic neurological disorder positively associated with the HLA-DQB1*06:02 allele and characterized by targeting hypocretin neurons [81][128]. Cross-reactive T cells for hypocretin autoantigen (HCRTNH2) and flu HA (H1N1 2009 strain) antigens were shown to influence narcolepsy development [82][129]. T cell cross-reactivity between viral epitope neuraminidase (NA175–189) and self-epitope protein-O-mannosyltransferase 1 (POMT1675–689) is also involved in narcolepsy pathogenesis [83][130].
6. The Role of Protective MHC-II Alleles in the Development of Autoimmune Diseases
Certain MHC alleles provide so-called “protection” toward the development of immune-related diseases due to peculiarities of antigen presentation, which affect the engagement of T cells [84][85][143,144]. “Protective” allele means that the risk of AD initiation in individuals carrying this allele is statistically lower than that for healthy donors, representing the average population [86][87][88][89][145,146,147,148]. Interestingly, the protein products of MHC-II protective alleles often differ from the products of risk alleles only by several amino acid residues localized in the TCR contact sites or near the key positions of the peptide-binding groove [90][149]. Supposedly, the protective effect can be ensured due to the deletion of autoreactive T cells in the thymus and/or induction of Treg cells (Figure 67A).
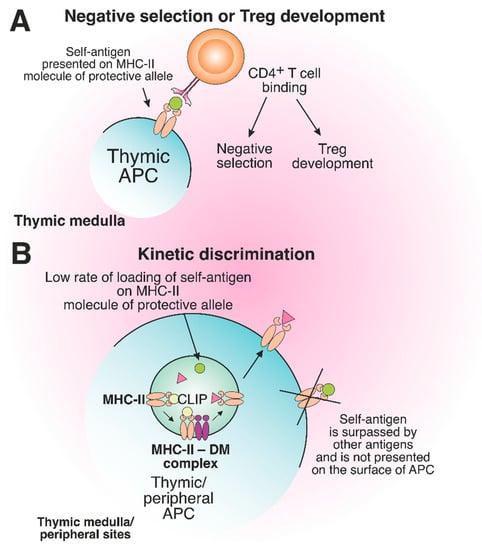
Figure 67. The role of protective MHC-II molecules in the suppression of autoreactive T cell development. (A) Recognition of the self-antigen presented on the product of the protective allele by CD4+ T cell results in negative selection or Treg development in the thymus. (B) The slow loading rate of self-antigen on the MHC-II molecule of the protective allele results in the failure of pMHC complex assembly on the surface of thymic or peripheral APCs.
