Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Nirmal Mazumder and Version 2 by Rita Xu.
Cancer is one of the dreaded diseases to which a sizeable proportion of the population succumbs every year. Circulating tumour cells (CTCs) have grabbed the attention of researchers in the detection of metastasis and there has been a huge surge in the surrounding research activities. Acting as a biomarker, CTCs prove beneficial in a variety of aspects. Nanomaterial-based strategies have been devised to have a tremendous impact on the early and rapid examination of tumor cells.
- circulating tumour cells
- metastasis
- nanotechnology
- nanomaterials
1. Introduction
Cancer is a broad term that often refers to a set of diseases characterized by uncontrolled cell growth with the ability to spread throughout the animal body [1]. It is one of the major causes of mortality globally, affecting patients from all socioeconomic backgrounds and their families. According to World Health Organization (WHO) statistics, 18.1 million new cases and 9.6 million deaths occurred in 2018 due to cancer, with secondary cancer or metastasis accounting for more than 90% of them [2][3][2,3]. The growing prevalence of cancer has encouraged the development of rapid and sensitive techniques for early detection. Currently, several methods, such as detecting the levels of metabolites or tumour markers in the blood, biopsy followed by histological evaluation, ultrasonography, magnetic resonance imaging, computerised tomography, positron emission tomography, and endoscopic-based examination are available [4]. The majority of the techniques are routinely used for cancer diagnosis and disease monitoring at a later stage only after the appearance of initial symptoms. As cellular and molecular sciences advance, various indicators and markers for early cancer detection are being investigated. Biomarkers based on gene and RNA expression are regularly used for the expeditious evaluation of cancers [5][6][7][5,6,7]. However, the requirements of extensive facilities and expensive instruments have limited its diagnostic application. On the other hand, dissemination of circulating tumour cells (CTCs) from primary tumours due to metastasis—which then spreads into the lymphatic nodes and blood, has been seen as an important marker for early diagnosis of cancer [8]. They stay either as a single cell or in a group, which can move from one site to another causing tumour [9] The early invasion of CTCs along with fast progression is vital for accurate monitoring, and detection of the tumour cells and an important part of liquid biopsy [10][11][10,11]. Detection and genomic characterization of CTCs can also help to investigate the mechanism of metastasis [12][13][12,13]. Liquid biopsy, often referred to as fluid biopsy or liquid phase biopsy, is a non-invasive diagnostic method for the analysis of non-solid biological samples such as blood. It overcomes the limitation of the inability to obtain heterogeneous information with traditional methods and helps in the identification of both genetic and epigenetic causes related to any diseases [14]. Liquid biopsy is not a routine test in clinical practice; however, over the past few years, it has evolved as a promising tool for the effective and early detection, monitoring of recurrence and evaluation treatment efficacy of cancer. The early description of circulating free DNA (cf DNA) and RNA in 1948, without knowing in detail about it, is said to be the first step towards the biopsy [15]. Ever since, various other biomarkers have also been looked into, for effective disease management (Figure 1). Micro-RNA, cf DNA, circulating tumour DNA, metabolites, CTCs, exosomes and other extracellular vesicles are some of the markers which have been significantly worked upon. Researches isolate these markers from blood and analyze them by different biochemical and analytical methods such as next-generation sequencing, polymerase chain reactions and mass spectrometry-based genotyping assay [7][16][7,16]. With the advancement of cancer treatment towards a shift to personalized medicine, this developing technology has the potential to change and/or complement functional cancer care. The advancement of liquid biopsy procedures has resulted in the incorporation of extremely effective approaches for the rapid detection and separation of CTCs [17], with nanotechnology at the forefront. Due to the unique physicochemical properties of nanomaterials emerging from their high surface area, shape, size, and optical nature, nanotechnology has proven to be the most reliable strategy in cancer diagnosis [18]. Higher surface-to-volume ratio property allows strong binding of the ligands which helps in recognizing critical biomarker molecules. As such, with the advent of this strategy, CTCs isolation and detection with higher efficiency and specificity, even at extremely low numbers in the circulatory system, has recently been achievable, allowing for early cancer diagnosis [19].
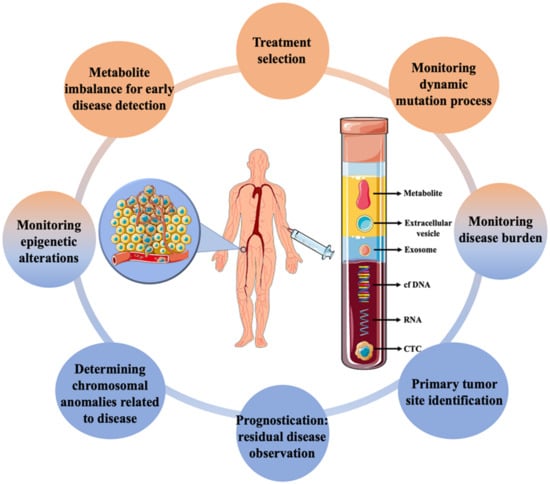
Figure 1. Applications of liquid biopsy in cancer diagnosis along with the different biomarkers commonly targeted using the technique.
2. Biogenesis of CTCs
CTCs are a rare subset of cells that are released from primary tumours and act as the precursor for the growth of additional cancerous tissue in a different location in the body [20]. The hallmarks of cancer such as invasiveness and motility give rise to the ability of malignant tumours to be metastatic. This is devised to be the main factor for the formation and release of CTCs in the circulatory system [21]. Though the formation and progression of cancer is a complex and little understood process, with the development of modern tools, this can be explored. The metastasis and carcinogenesis of tumour cells are commonly described to be a multi-step process, as shown in Figure 2 [22]. Initially, the cell loses its normal cell cycle ability, whether being triggered by internal factors or due to extremal stimulus. This causes the tumour cells to accumulate numerous mutations, prompting them to become malignant [23]. In most cases, it is not the original tumour that causes fatalities, but the spread of malignant cells to other organs. Once the primary tumour grows to a certain size, the available nutrients and oxygen become limited for the continuously dividing cells. This triggers the formation of hypoxia-inducing factors (HIFs). HIFs, with other pathways in the tumours microenvironment, trigger the expression of angiogenesis-promoting factors such as VEGF and angiopoietin-1 and 2, promoting neovascularization around the tumour [24][25][24,25]. Further, the expression of HIFs, angiogenesis, and other factors such as laminin have been shown to reduce the expression of E-cadherin through the pleiotropic effect [26]. The suppressed expression of E-cadherin in the tumour microenvironment reduces cell-to-cell adhesion and increases the mobility of the tumor cells. This begins the process of epithelial-to-mesenchymal transition (EMT). It is observed that changes in the expression of makers and molecules on its surface, such as altered expression of extracellular matrix (ECM)-cell adhesion molecules, suppress expression of epithelial markers, and increase expression of mesenchymal markers [27][28][27,28]. EMT is often considered to be the most accepted hypothesis regulating the formation of CTCs. However, this process is highly complex and still unknown. After the EMT process, the tumour cells adopt a motile, invasive phenotype leading to their detachment from the primary tumour and migration into the neighboring tissue, also known as local invasion. The process is regulated by complex mechanistic changes in cytoskeleton structure, proteolysis of surrounding tissue and alteration in cell–ECM dynamics [29]. Thereafter, some of the cancer cells evade into the network of blood vessels formed around the primary tumor body by intravasation. This process allows cancer cells to initiate metastases at a distant, different locations within the animal body. The cancer cells in the circulation (CTCs) have the ability to evade anoikis due to their mesenchymal properties and also due to the expression of anoikis inhibitors (such as XIAP) in the sub-populations of the cells, thereby resulting in the cells being resistant to apoptosis [30]. The CTCs in circulation are believed to be non-proliferative in nature and travel around the body until they adhere to the capillary bed of a different organ due to their molecular properties or size. Therefore, it is observed that secondary tumours can be formed at distant organs from the original site of the primary neoplasm causing the most fatal malignancies [31]. In addition, it is worth mentioning that these cancer cells also take part in local metastasis, thereby often reaching the lymph nodes that make the disease more severe. Once the cancer cells are attached to the capillary surface, they penetrate the endothelial layer of the blood vessel, thereby moving into the host organ. Then the cells undergo another round of phenotypic alteration almost similar to EMT which is known as mesenchymal to epithelial transition [32][33][32,33]. Though this process is less understood and is still being studied, it is observed that the cancer cells regain their epithelial characteristics, which helps them to acclimatize to new surroundings. Apart from the EMT-mediated motility of cancer cells in the circulation, the non-EMT-associated scenarios have also been known to give rise to CTCs. Centrosome amplification is one of them, in which the E-cadherin expression is maintained throughout the process of CTC formation. The centrosome is sought to play the role of organizing flagella and cilla, apart from also facilitating the separation of chromosomes. Thus, the centrosome has been shown to induce the propagation and motility of cancer cells [34]. Another method is by the non-EMT-mediated dissemination to form CTCs clusters, in which 2–50 cells are clustered together by intracellular adhesion as they move across the vessels. These cells have a higher probability of surviving anoikis than individual circulating cancer cells [35]. This contributes to a better sustainable metastasis and increases the likelihood of extravasation in secondary sites. As not all CTCs lose their epithelial properties, it is also necessary to consider these properties for the effective detection of cells in the blood. It should also be noted that cancer cells might enter the blood following the surgical or microscopic procedures to remove the tumour (such as in case of minimum residual disease) in addition to the naturally occurring CTCs from primary or secondary neoplasms [36][37][36,37].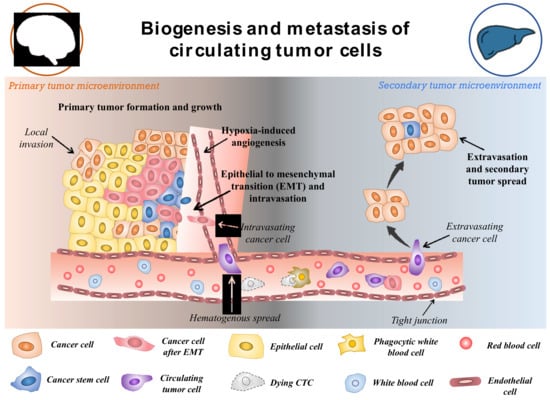
Figure 2. Schematic representation of potential biogenesis and the transport mechanism of circulating tumor cells. Reproduced from [38].
3. Nanotechnology
Nanotechnology has been utilized in various biological research to carry out with high-throughput, sensitivity, selectivity, and specificity and it possesses a high capacity to measure multiplexed systems. Due to their smaller size, they possess a high surface-to-volume ratio, which increases their efficiency towards cellular binding capacity; they are utilized in cancer research which has led to the advancement in significant detection, isolation and enrichment of CTCs from the blood sample. The nanoparticles that are implemented in the analysis of CTCs are classified into various types depending upon their type, shape, and structure. Some of them include quantum dots/fluorescent nanoparticles, surface-enhanced Raman scattering nanoparticles and many more. Each of them has its unique advantages and disadvantages, as discussed in Table 1, which make them suitable for cancer research.Table 1. Different types of nanomaterials used for the detection of CTCs.
| Types of Nanoparticles | Properties | Advantages | Utilization in CTC Analysis | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Quantum Dots | Tunable narrow fluorescence emission Large absorption coefficient High brightness High photostability High quantum yield Longer fluorescence lifetime |
High specificity High sensitivity |
Detection of CTCs with high metastatic potential Quantified detection of CTCs in the blood vessel. |
[53][54][55][56] | [53,54,55,56] | ||||||
| SERS Nanoparticle | Requires a single source of excitation Display minimized photobleaching |
Helps in efficient detection of even a single cell Development of ultrasensitive probes with high specificity | Enrichment, detection, multicolour imaging and enumeration of CTCs | [55][57][58][59] | [55,57,58,59] | ||||||
| Magnetic Nanoparticle | High cellular binding capability Outstanding stability in blood Capability to attach large number of MNPs to a single cell without any aggregation. |
Low cost High stability |
Immunomagnetic Separation, enrichment, and detection of CTCs | [55][60][61] | [55 | [ | ,60 | 62 | ,61 | ] | ,62] |
| Conductive Nanoparticles/CNTs | Extraordinary mechanical strength High electrical conductivity Conductivity varies with change in the chemical binding |
Extraordinary electrical conductivity Outstanding heat conductivity |
Electronic detection of CTCs with low expression of protein without any enrichment process by real-time electrical impedance sensing method | [54][55][63][64] | [54,55,63,64] | ||||||
| Upconversion Nanoparticles | Strong and sharp emission spectra High penetration depth Large Stokes shifts Low background signal High resistance to photobleaching |
High photo stability High thermal stability |
High sensitivity bio-detection and imaging of CTCs | [54][55][[67] | [54 | 65][66] | ,55,65,66,67] | ||||
| Metallic nanoparticles/Plasmonic nanoparticles | Collective coherent oscillation of the electrons at resonance High amount of absorption and scattering of light at resonance |
Biocompatibility High chemical stability Minimum toxicity |
Colometric based detection of CTCs in blood | [54][[70] | [54 | 68][69] | ,68,69,70] |
3.1. Quantum Dots (QDs)/Fluorescent Nanoparticles
QDs are zero-dimensional fluorescent nanoparticles, where all the dimensions of the particles are less than 100 nm. They possess a semiconductor core covered by a shell as shown in Figure 3a. They are generally made up of elements from groups II to VI or III to V of the periodic table. They pose discrete energy levels with varying bandgap depending upon their size, which ranges between 2–10 nm, which is less than the size of the exciton Bohr radius. They display unique electronic and optical properties such as higher quantum yield, longer fluorescence lifetime, large absorption coefficient, high-quality brightness, excellent photostability, and narrow and tunable fluorescence emission which lies between visible to infrared wavelengths. Due to these properties, QDs have led to the development of modern-day probes which have high specificity and sensitivity [53][54][55][56][71][53,54,55,56,71].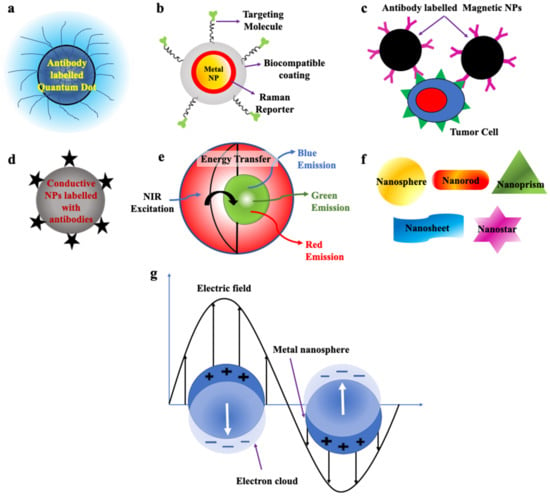
Figure 3. Schematic representation of (a) Quantum dot labelled with antibodies (Redrawn after [72]). (b) SERS nanoparticles (Redrawn after [73]). (c) Magnetic Nanoparticles labelled with antibodies (Redrawn after [62]). (d) Conductive nanoparticles. (e) Upconversion nanoparticles (Redrawn after [74]). (f) Nanoparticles of various morphologies used to detect CTCs. (g) Metallic nanoparticle displaying surface plasmon resonance (Redrawn after [75]).
