Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 5 by Jessie Wu.
Cancer treatments have undergone significant advances, although they are not exempt from side effects, including skin toxicity. Different studies show that skin care for cancer patients can be effective in reducing sequelae such as inflammation, xerosis, skin rash, and radiodermatitis, among others. It is necessary to implement measures that improve the patient’s well-being and, therefore, thalassotherapy techniques and the marine environment could be an effective resource to achieve this goal. Thalassotherapy is the combined use of marine elements (water, algae, mud and climate), in a marine environment for healing and well-being improvement purposes.
- cancer
- thalassotherapy
- skin care
1. Sea Water: Effects on Skin
The composition of seawater is similar in all oceans and seas, with variations in component concentration. Table 1 shows the average composition of seawater in the world, expressed in mg/L [1].
Table 1. Seawater composition: major ion concentration (mg/L) in seawater around the world.
| Parameter | Worldwide Average |
|---|---|
| Chloride, Cl− | 18,980 |
| Sodium, Na+ | 10,556 |
| Sulphate, SO4−2 | 2649 |
| Magnesium, Mg+2 | 1272 |
| Calcium, Ca+2 | 400 |
| Potassium, K+ | 380 |
| Bicarbonate, HCO3− | 140 |
| Bromide, Br− | 65 |
| Borate, H2BrO3− | 26 |
| Strontium, Sr+2 | 13 |
| Fluoride, F− | 1.0 |
| TDS | 34,482 |
(TDS: Total Dissolved Solids).
Seawater is very well known for its curative effects in the treatment of skin diseases such as eczemas, dermatoses, psoriasis, respiratory problems such as nasopharyngeal inflammations, gynecological diseases such as vaginitis, and other infections of the external genital organs. For skin care, it has been used as a moisturizing agent; it also regularizes the sebaceous production, avoiding the consequent formation of scaling of the scalp (dandruff). It is worth to mention the important role of seawater in the absorption of saline and metallic ions, favoring the excretion of toxic residues and a certain oxygenation of tissues [2].
Seawater has been shown to have important effects on skin hydration and regeneration, although there are few scientific studies, since mostly testimonials are available. Most of the studies focus on the hypersaline water of the Dead Sea; a few of them examine the seawater of the Atlantic coasts and others do the deep seawater.
From the studies of the last ten years, those carried out by Yoshikawa et al. [3][4] deserve to be cited. In the first study, the effects of sea water and its main components on experimental irritant contact dermatitis induced by sodium lauryl sulphate (SLS) cumulative irritation were investigated, and it was concluded that the effect of sea water may be attributed to skin barrier preservation by NaCl and KCl, and an emollient effect by NaCl [3]. In the second study, the effects of three types of mineral water with NaCl and KCl with different concentrations (500 nM NaCl + 10 nM KCl; 250 nM NaCl + 10 nM KCl, and 250 nM NaCl + 50 nM KCl) were studied in order to assess the most effective water concentration to prevent disruption of skin barrier. The authors suggested that mineral water with 250 mM of NaCl and 50 mM of KCl may be useful as adjunctive therapy in atopic dermatitis and other chronic dermatitis [4].
Bathing in the Dead Sea waters (with an average concentration of 280 g/K) has been extensively studied: magnesium salts are known to bind water, influence epidermal proliferation and differentiation, and enhance permeability barrier repair [5]. In a study by Proksch et al. [6], immersion in magnesium-rich salt solutions from the Dead Sea was investigated (Mavena® Dermaline Mg Dead Sea). The experiment consisted of 15 min bathing in a solution containing 5% Dead Sea salt daily for 6 weeks; transepidermal water loss (TEWL), stratum corneum hydration, and skin redness in atopic dry skin (xerosis) were measured, concluding that bathing with Mavena® Dermaline Mg Dead Sea salt solution, owing to its high content of magnesium ions, enhanced stratum corneum hydration, improved skin barrier function and reduced skin roughness and inflammation [6].
On the other hand, as has been mentioned, in recent years, the effect of deep-sea water on different skin disorders has been studied. A study in NC/Nga mice model carried out by Bak et al. [7] with mineral water from deep-sea bedrock, rich in minerals such as Ca, Mg, Na, K, Fe, and others, investigated the preventive effects of natural deep-sea water on developing Atopic dermatitis, concluding that this water inhibits the development of atopic dermatitis-like skin lesions [7]. Lee et al. [8] studied, in a skin equivalent model, Jeju lava sea water, showing that this water (rich in sodium, magnesium and calcium) increased the CD44 (hyaluronic acid receptor) which is related to skin hydration; the authors conclude that the Jeju lava sea water may help to improve skin hydration [8].
Moreover, Chun et al. [9] studied the effect of deep-sea water (high levels of Mg and Ca) on lipopolysaccharide (LPS)-induced inflammatory response in RAW 264.7 macrophage cells and determined that this water suppressed inflammatory responses via the MAPK/AP-1 and NF-κB Signaling Pathway [9]. Recently, Lee et al. [10] evaluated the effects of mineral-balanced deep-sea water (DSW) on Atopic dermatitis-like skin damage both in vitro and in vivo. The results showed that DSW is effective in the treatment and prevention of atopic-type skin lesions. The mechanism of action involved seems to be the suppression of the expressions of proinflammatory chemokines and cytokines, as well as the inhibition of histamine and IgE production underlying the STAT1 and JNK1/2 signaling pathways. In addition, other mechanisms involved are the positive regulation of filaggrin and the recovery of involucrin expressions and the inhibition of IL-4 production, which help restore skin health. The authors suggested that mineral-balanced DSW may be useful in preventing and treating skin inflammation caused by skin disorders, including atopic dermatitis [10].
On the other hand, Carbajo et al. [11] revised the mechanism of action specific to saline waters; when topically administered, this water rich in sodium and chloride penetrated the skin where it was able to modify cellular osmotic pressure and stimulate nerve receptors in the skin via cell membrane ion channels known as “Piezo” proteins. They postulated that the effects of salt mineral waters are mediated by mechanisms linked to the concentration and type of salts involving cellular osmosis-mediated activation/inhibition of cell apoptotic or necrotic processes, and in turn, this osmotic mechanism modulates the mechanosensitive piezoelectric channels [11].
Another aspect of great interest in the use of seawater is wound healing. In a study carried out by Huynh et al. [12], it was demonstrated that short-term rinsing with NaCl promoted hGFs migration and increased the expression of extracellular matrix as well as cytoskeletal proteins; the authors concluded that these data strongly support the empirical use of NaCl mouth rinse [12]. Cantore et al. [13], in a single-blinded randomized controlled trial, studied the effects of sea salt mouth rinse on subjects undergoing oral surgery; the results showed an appreciable wound healing in the experimental group when compared to the control, with no reported adverse effects [13]. Another study demonstrated that the use of a sea salt-based mouthwash in daily oral hygiene reduces the bacterial levels of Streptococcus mutans linked to the combined action of xylitol and lysozyme, together with the action of sea salt [14]. In addition, another research showed that soaking in 7% of table salt concentration can significantly accelerate the wound healing process compared to the control group, with a decrease in wound diameter on the 3rd day and complete healing on the 7th day [15].
2. Procedures and Techniques of Thalassotherapy
Seawater is applied to the body using different respiratory (aerosol) or topical techniques such as bathing and pressure techniques, whether partial (legs and feet, arms and hands) or full body, as well as algae and marine mud applications. The so-called complementary techniques are also used, which include saunas and steam baths, mechanical techniques, electrotherapy or different types of massages. The most used techniques are summarized in Table 2 and some examples are shown in Figure 1A–C.

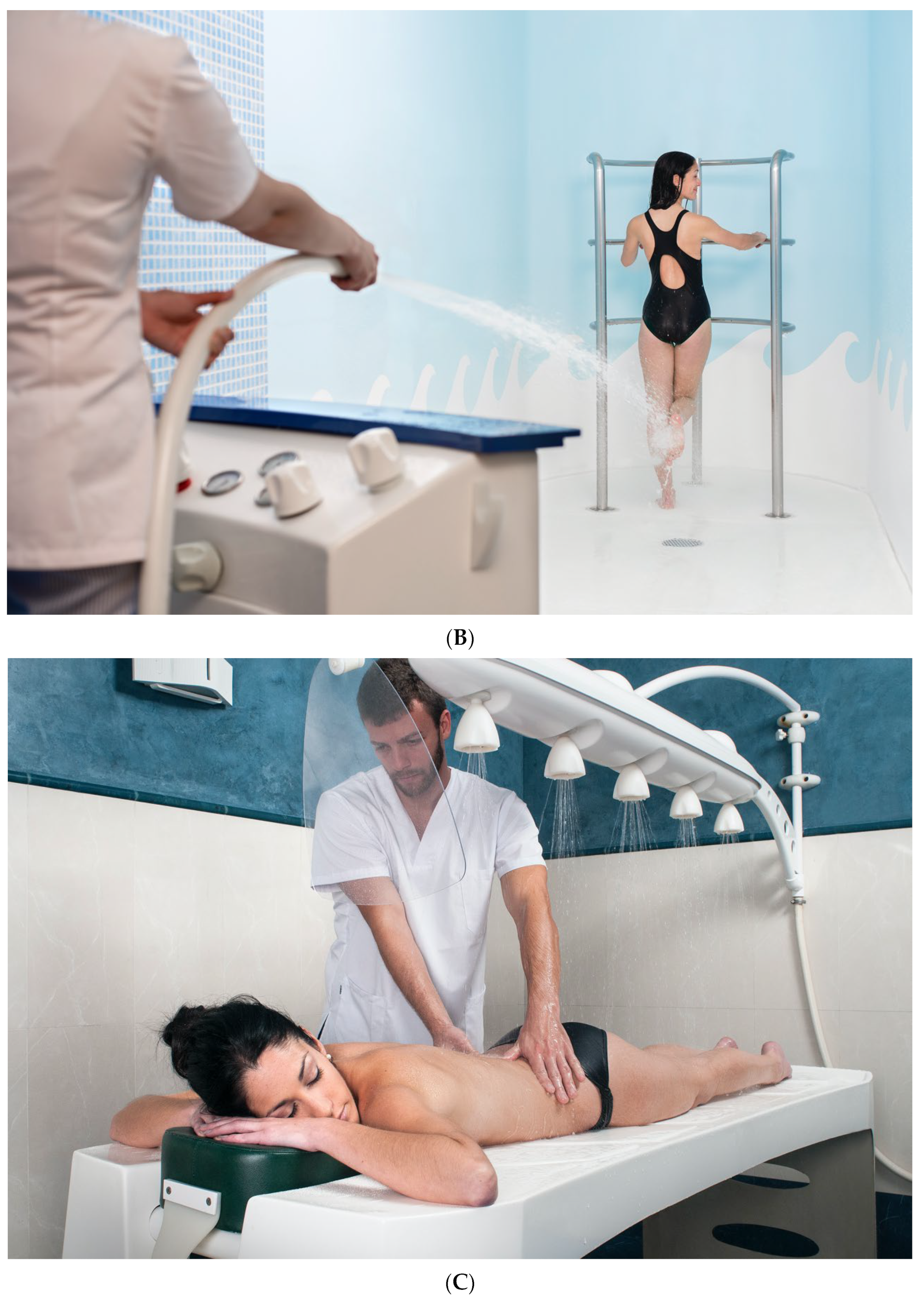
Figure 1. Thalassotherapy techniques: (A) Hydromassage bath; (B) jet shower; (C) Vichy shower (Courtesy of Talaso Atlántico, Spain).
Table 2. Procedures and techniques of thalassotherapy.
| Thalassotherapy Techniques | Other Complementary Techniques |
|---|---|
| Aerosols | Sauna and steam baths |
| Bubbling bath | Pressotherapy |
| Hydromassage bath | Electrotherapy |
| Underwater massage | Thermo- and Criotherapy |
| Jet showers | Lymphatic draining |
| Affussion showers | Massages (therapeutic, antistress, etc.) |
| Vichy showers | Osteopathy |
| Scottish showers | Oriental massages (Shiatsu, Thai, etc.) |
| Phlebologic path | Yoga, Tai-chi and others |
| Hand and Foot Baths | Reeducational therapies |
| Hydrotherapy pool | Nutritional therapies and counselling |
| Seaweed wrappings | Water therapies: Watsu, Ai Chi, etc. |
| Marine mud applications | Aquagym, Aquabike, etc. |
Added to this is the marine environment, used in the treatment of non-allergic bronchial asthma and also in psoriasis [16][17] and, in certain cases, the use of heliotherapy [18].
3. Thalassotherapy and Post-Oncological Recovery
There is sufficient scientific evidence on the benefits of hydrotherapy techniques with seawater and mud or marine mud in functional rehabilitation [19][20][21][22][23]. Hydrotherapy treatments carried out with ordinary water show the benefits in the rehabilitation of different traumas and joint pathologies, as well as hydrothermal techniques carried out with thermal spring water [24]. Some studies whose conclusions could be extrapolated to rehabilitation and recovery after an intervention or oncological therapy are described below.
Dalenc et al. [25], in a randomized, multicenter controlled study 1–5 weeks after completing radiotherapy, investigated the efficacy of post-treatment hydrotherapy as supportive care for management of persistent/long-lasting dermatologic adverse events (dAEs). Both groups received supportive care and the intervention group received 3 weeks of specific hydrotherapy. The study demonstrated that a 3-week supportive care program with hydrotherapy initiated after completion of radiotherapy is an effective and safe supportive treatment and is of significant benefit to these patients resulting in an improvement of dAEs, therefore reducing the impact of breast cancer and its treatment on overall physiological well-being and dermatological and health-related QoL [25].
Likewise, there are experiences of care and recovery of cancer survivors in thermal spa centers in which the main base is also the hydrothermal techniques and the effect of the minerals and trace elements of the mineral–medicinal waters in the organic balance [26][27]. Thus, in 2005, Strauss-Blachet et al. [28] conducted a study on the efficacy of adding spa therapy to a rehabilitation program after breast cancer. One hundred forty-nine women, 32 to 82 years, participated in a study 3 to 72 months after breast cancer surgery; quality of life (QoL, EORTC QLQ-C30), anxiety, and depression (HADS) were measured 2 weeks before, at the end, and 6 months after rehabilitation; the tumor maker CA 15-3 was measured at the beginning, end, and at 6-month follow-up. Patients received an individualized rehabilitation program incorporating different hydrothermal techniques such as carbon dioxide baths and mud packs, as well as other complementary ones such as manual lymph drainage, massages, exercise therapy, psychological counseling, and relaxation training. The results showed that quality of life and mood improved significantly, the greatest short-term improvements were observed for mood-related aspects of quality of life, the most lasting improvements detected for physical complaints (e.g., fatigue) [28].
Another study on oncological patients showed that spa therapy increased clusterin serum concentration (a stress-associated cytoprotective glycoprotein involved in many physiological and pathophysiological processes which is up-regulated by various apoptotic triggers in many cancers and neurodegenerative diseases). Authors suggested that this result is probably due to the positive effects of balneotherapy; however, the sample was very small and further research is required [29].
Galvez et al. revised the effects of balneotherapy considering it a clinically effective complementary approach in the treatment of low-grade inflammation- and stress-related pathologies; the physiological effects are exerted through both physical mechanisms—mainly linked to heat therapeutic effects—and chemical and biological properties of the agents. Therefore, thermotherapeutic effects are the basis of these treatments with a range of temperatures from 38 to 42 °C. It is well known that severe heat stress leads to cellular damage and cell death, and mild heat stress induces heat shock response which protects cells and organisms from severe damage, allows resumption of normal cellular and physiological activities. In this resviearchw, the authors postulate that hormesis can play a role in the biological effects of balneotherapy, and these effects can be related to non-specific factors such as heat—which induces the heat shock response, and therefore the synthesis and release of heat shock proteins—and also to specific biochemical components such as hydrogen sulfide (H2S) in sulfurous water and radon in radioactive water [26].
Kwiatkowski et al. [30][31] studied the improvement of the quality of life of breast cancer survivors with 2 weeks of physical and educational intervention in thermal spa centers, concluding that balneotherapy is effective in improving the quality of life and that the effects are maintained for at least 6 months, recommending a second intervention after that time.
Furthermore, another study investigated the effectiveness of a warm-water footbath on relieving fatigue and insomnia problems in patients undergoing chemotherapy. Two groups were investigated (control and experimental group) in a longitudinal study design. Women diagnosed with gynecologic cancer and receiving a 4-series platinum chemotherapy regimen were recruited and then followed up for 6 months. They completed fatigue and insomnia items on the 1st, 2nd, 4th, 7th, and 14th day after each scheduled chemotherapy. Participants in the experimental group soaked their feet in 41 °C to 42 °C warm water for 20 min every evening, starting from the eve of receiving the first chemotherapy, whereas participants in the comparison group did not do so. The results showed that participants in the experimental group reported a significant reduction in fatigue and improvement in sleep quality from the second session of chemotherapy and continued to improve during the study period [32].
Recovery post-breast cancer was also studied; Cantarero-Villanueva et al. [33] carried out a study with 40 women aged 29–71 years with stage I–III breast cancer who reported arthralgia. The hydrotherapy intervention consisted of 24 sessions 3 days a week over 2 months; each session included 5 min of warm-up, 15–20 min of aerobic exercise, 15 min of mobility exercise, and 20 min of recovery techniques. The results showed that participants experienced a decrease in pressure pain threshold measured in neck, hand, shoulder and leg, as measured by algometry pressure and waist circumference; body mass index and cancer-related fatigue did not show significant improvement [33].
Later on, a randomized controlled trial was conducted by the same research group in breast cancer survivors. They compared two groups of patients: the experimental group followed aquatic exercise in deep water pool and the control group followed the usual care; the intervention group attended aquatic exercise sessions 3 times per week for 8 weeks in a heated deep swimming pool (sessions were 60 min in duration: 10 min of warm-up, 40 min of aerobic and endurance exercises, and 10 min of cool-down exercises). Patients allocated to the usual care group followed the oncologist’s recommendations in relation to a healthy lifestyle. Values for fatigue (Piper Fatigue Scale), mood state (Profile of Mood States), and abdominal (trunk curl static endurance test) and leg strength (multiple sit-to-stand test) were collected at baseline, after the last treatment session, and at a 6-month follow-up. Immediately after discharge, the aquatic exercise group showed a large effect size in total fatigue score, trunk curl endurance, and leg strength, but negligible effects in vigor, confusion, and disturbance of mood. At the conclusion of the 6-month follow-up period, the aquatic exercise group maintained large to small effect sizes in fatigue scores, multiple sit-to-stand test, and trunk curl static endurance and negligible effects for the fatigue severity dimension and different scales of the Profile of Mood States. The authors concluded that an aquatic exercise program conducted in deep water was effective for improving cancer-related fatigue and strength in breast cancer survivors [34].
Mourgues et al. [35], in a multicenter randomized controlled trial with and intervention group that included women in complete breast cancer remission without contraindication for physical activities or cognitive disorders, undergoing spa therapy and nutritional consultation, confirmed that spa treatment is a cost-effective strategy to improve resumption of occupational and non-occupational activities and the abilities of women in breast cancer remission [35]. Finally, it is worth mentioning that in a review carried out by Reger et al. [36], several studies are detailed showing the efficacy of aquatic hydrotherapy for the improvement of lymphedema.
Therapies in the Dead Sea are an example of dermatological treatments with highly mineralized waters; in addition to treating psoriasis and atopic dermatitis, skin recovery treatments have been carried out on cancer patients. In a phase II study, researchers compared the outcomes of 24 treated patients with Dead Sea water and 30 conventionally treated patients matched for age, tumor site, and type of treatment. The Dead Sea products comprised a mouthwash solution (Lenom®) and a skin cream (Solaris®) used three times daily for 1 week before, during, and up to 2 weeks after completion of radiotherapy. Mucositis and dermatitis were evaluated using common toxicity criteria. The two Dead Sea products were shown to decrease skin and mucosal toxicity in head and neck cancer patients receiving radio-chemotherapy [37].
It is also worth mentioning the studies carried out with thalassotherapy treatments in fibromyalgia in which an improvement in fatigue and sleep disturbances were observed, aspects that are common to some of the side effects that occur after oncological therapies. These results can show the beneficial use of the thalassotherapy for the rehabilitation of cancer survivors [38].
Another study attempted to demonstrate the effects of the combination of thalassotherapy treatments (hydrotherapy with sea water) with sleep management and its influence on sleep, mood states, well-being, health outcomes and cognitive (sustained attention) and physical capacities in healthy middle-aged workers. Despite a short period of treatment (3 days) and a small group (11 participants), the results showed beneficial effects of 3 days of thalassotherapy care combined with sleep management for mood states, well-being, health outcomes, and cognitive and physical capacities in the general working population. The authors suggested that hydrotherapy care (3 days with two 2-h sessions of care) and sleep management resulting in increased total sleep time (TST) in the second night may explain immediate positive effects on self-reported psychological outcomes and objectively measured vigilance attention but also delayed positive effects on lower limb flexibility. It also would be interesting to evaluate the effectiveness of thalassotherapy in the prevention and management of professional burnout that has been previously described as associated with anxiety and sleep disorders [39]. In addition, thalassotherapy with deep-sea water was shown to be effective in recovery from fatigue and muscle damage [40].
As mentioned above, some thermal centers have implemented programs for post-cancer patient care; such is the example of Avène, La Roche-Posay, Balaruc-les-Bains, Saint-Gervais, La Bourboule or Uriage, some specialized in lymphedema such as Luz-Saint-Sauveur. Others focus on programs for the treatment of chronic diseases [41], which may also be of interest for cancer patient care.
Although there are no publications on the results of treatment programs for post-cancer patients, thalassotherapy centers have also joined this initiative. Table 3 lists some of them, which are mainly located in France.
Table 3.
Post-oncologic care programs in thalassotherapy centers.
| Thalassotherapy Center | Program Web Page |
|---|---|
| Thalasso Deauville | https://www.thalasso-deauville.com/en/12-day/125-post-cancer-stopover.html (accessed on 25 November 2022) |
| Atlanthal Hotel & Thalasso | https://www.atlanthal.com/es/curas/178-thalasso-post-cancer.html (accessed on 25 November 2022) |
| Roscoff-Hôtel Valdys, Beau Rivage Thalasso & Spa | https://www.thalasso.com/destination/roscoff (accessed on 25 November 2022) |
| Hôtel Thalasso & Spa Emeria Dinard | https://www.emeriadinard.com/ (accessed on 25 November 2022) |
| Thalasso Concerneau | https://www.concarneau-thalasso.com/ (accessed on 25 November 2022) |
| Côte Thalasso Banyuls-sur-Mer | https://www.cote-thalasso.fr/banyuls-sur-mer/cures (accessed on 25 November 2022) |
| Hotel Talaso Atlántico | https://www.talasoatlantico.com/es/talaso.html (accessed on 25 November 2022) |
Another aspect of great interest in the care of cancer survivor patients is physical activity (either through active exercise or relaxation activities such as yoga) and adequate nutrition [42][43][44][45][46]. In whose programs, thalassotherapy centers can also be main actors. Likewise, although scarce, there are studies on the influence of phototherapy for the immunological improvement of cancer patients [47], an aspect that may be of interest in thalassotherapy centers where heliotherapy is applied.
On the other hand, nowadays, more and more importance is assigned to healing environments (understanding that the environment of a thalassotherapy center has healing effects), highlighting cures in natural environments; by way of example, the studies by Ray et al. [48] assert that the natural environment can counteract attentional fatigue in recently diagnosed breast cancer survivors, and therapeutic landscapes can reduce the state of anxiety, improving the health of survivors. Likewise, Liamputtong and Suwankhong [49] also described the importance of healing landscapes for the “the emotional healing”.
References
- Drioli, E.; Giorno, L.; Fontananova, E. Comprehensive Membrane Science and Engineering; Elsevier Science & Technology: Oxford, UK, 2017; ISBN 9780444637758.
- Pereira, L. Seaweeds as Source of Bioactive Substances and Skin Care Therapy—Cosmeceuticals, Algotheraphy, and Thalassotherapy. Cosmetics 2018, 5, 68.
- Yoshizawa, Y.; Tanojo, H.; Kim, S.J.; Maibach, H.I. Sea water or its components alter experimental irritant dermatitis in man. Skin Res. Technol. 2001, 7, 36–39.
- Yoshizawa, Y.; Kitamura, K.; Kawana, S.; Maibach, H.I. Water, salts and skin barrier of normal skin. Skin Res. Technol. 2003, 9, 31–33.
- Schempp, C.M.; Dittmar, H.C.; Hummler, D.; Simon-Haarhaus, B.; Schöpf, E.; Simon, J.C.; Schulte-Mönting, J. Magnesium ions inhibit the antigen-presenting function of human epidermal Langerhans cells in vivo and in vitro. Involvement of ATPase, HLA-DR, B7 molecules, and cytokines. J. Investig. Dermatol. 2000, 115, 680–686.
- Proksch, E.; Nissen, H.-P.; Bremgartner, M.; Urquhart, C. Bathing in a magnesium-rich Dead Sea salt solution improves skin barrier function, enhances skin hydration, and reduces inflammation in atopic dry skin. Int. J. Dermatol. 2005, 44, 151–157.
- Bak, J.-P.; Kim, Y.-M.; Son, J.; Kim, C.-J.; Kim, E.-H. Application of concentrated deep sea water inhibits the development of atopic dermatitis-like skin lesions in NC/Nga mice. BMC Complement. Altern. Med. 2012, 12, 108.
- Lee, S.H.; Bae, I.-H.; Min, D.J.; Kim, H.-J.; Park, N.H.; Choi, J.H.; Shin, J.S.; Kim, E.J.; Lee, H.K. Skin Hydration Effect of Jeju Lava Sea Water. J. Soc. Cosmet. Sci. Korea 2016, 42, 343–349. (In Korean)
- Chun, S.-Y.; Lee, K.-S.; Nam, K.-S. Refined Deep-Sea Water Suppresses Inflammatory Responses via the MAPK/AP-1 and NF-κB Signaling Pathway in LPS-Treated RAW 264.7 Macrophage Cells. Int. J. Mol. Sci. 2017, 18, 2282.
- Lee, K.-S.; Chun, S.-Y.; Lee, M.-G.; Kim, S.; Jang, T.-J.; Nam, K.-S. The prevention of TNF-α/IFN-γ mixture-induced inflammation in human keratinocyte and atopic dermatitis-like skin lesions in Nc/Nga mice by mineral-balanced deep sea water. Biomed. Pharmacother. 2018, 97, 1331–1340.
- Carbajo, J.M.; Maraver, F. Salt water and skin interactions: New lines of evidence. Int. J. Biometeorol. 2018, 62, 1345–1360.
- Huynh, N.C.-N.; Everts, V.; Leethanakul, C.; Pavasant, P.; Ampornaramveth, R.S. Rinsing with Saline Promotes Human Gingival Fibroblast Wound Healing In Vitro. PLoS ONE 2016, 11, e0159843.
- Cantore, S.; Ballini, A.; Saini, R.; Altini, V.; De Vito, D.; Pettini, F.; DiPalma, G.; Inchingolo, F. Effects of sea salt rinses on subjects undergone to oral surgery: A single blinded randomized controlled trial. Clin. Ter. 2020, 170, e46–e52.
- Ballini, A.; Cantore, S.; Signorini, L.; Saini, R.; Scacco, S.; Gnoni, A.; Inchingolo, A.D.; De Vito, D.; Santacroce, L.; Inchingolo, F.; et al. Efficacy of Sea Salt-Based Mouthwash and Xylitol in Improving Oral Hygiene among Adolescent Population: A Pilot Study. Int. J. Environ. Res. Public Health 2020, 18, 44.
- Samidah, S.; Prihantono; Ahmad, M.; Jompa, J.; Rafiah, S.; Usman, A.N. The effectiveness of 7% table salt concentration test to increase collagen in the healing process of wound. Gac. Sanit. 2021, 35, S199–S201.
- Schuh, A.; Nowak, D. Evidence-based acute and long-lasting effects of climatotherapy in moderate altitudes and on the seaside. DMW—Dtsch. Med. Wochenschr. 2011, 136, 135–139.
- Ezhov, V. Climate-therapy at seaside resorts in modern medical and wellness practice. Vopr. Kurortol. Fizioter. Lech. Fiz. Kult. 2021, 98, 60–66.
- Munteanu, C.; Munteanu, D.; Hoteteu, M.; Dogaru, G. Balneotherapy—Medical, scientific, educational and economic relevance reflected by more than 250 articles published in Balneo Research Journal. Balneo PRM Res. J. 2019, 10, 174–203.
- Morer, C.; Michan-Doña, A.; Alvarez-Badillo, A.; Zuluaga, P.; Maraver, F. Evaluation of the Feasibility of a Two-Week Course of Aquatic Therapy and Thalassotherapy in a Mild Post-Stroke Population. Int. J. Environ. Res. Public Health 2020, 17, 8163.
- Hoteteu, M.; Romanian Association of Balneology; Munteanu, C.; Ionescu, E.V.; Almășan, R.E. Bioactive substances of the Techirghiol therapeutic mud. Balneo PRM Res. J. 2018, 9, 5–10.
- Antonelli, M.; Donelli, D. Thalassotherapy, Health Benefits of Sea Water, Climate and Marine Environment: A Narrative Review. In Proceedings of the 6th International Electronic Conference on Water Sciences, Babylon, Iraq, 15–30 November 2021.
- Eröksüz, R.; Forestier, F.B.E.; Karaaslan, F.; Forestier, R.; Işsever, H.; Erdoğan, N.; Karagülle, M.Z.; Dönmez, A. Comparison of intermittent and consecutive balneological outpatient treatment (hydrotherapy and peloidotherapy) in fibromyalgia syndrome: A randomized, single-blind, pilot study. Int. J. Biometeorol. 2020, 64, 513–520.
- Varzaityte, L.; Kubilius, R.; Rapoliene, L.; Bartuseviciute, R.; Balcius, A.; Ramanauskas, K.; Nedzelskiene, I. The effect of balneotherapy and peloid therapy on changes in the functional state of patients with knee joint osteoarthritis: A randomized, controlled, single-blind pilot study. Int. J. Biometeorol. 2020, 64, 955–964.
- Matsumoto, S. Evaluation of the Role of Balneotherapy in Rehabilitation Medicine. J. Nippon Med. Sch. 2018, 85, 196–203.
- Dalenc, F.; Ribet, V.; Rossi, A.; Guyonnaud, J.; Bernard-Marty, C.; de Lafontan, B.; Salas, S.; Royo, A.-L.R.; Sarda, C.; Levasseur, N.; et al. Efficacy of a global supportive skin care programme with hydrotherapy after non-metastatic breast cancer treatment: A randomised, controlled study. Eur. J. Cancer Care 2018, 27, e12735.
- Gálvez, I.; Torres-Piles, S.; Ortega-Rincón, E. Balneotherapy, Immune System, and Stress Response: A Hormetic Strategy? Int. J. Mol. Sci. 2018, 19, 1687.
- Massiero, S. Health Resort Medicine and Human Immune Response. How Balneology Can Protect and Improve Our Health. FEMTEC Editions. 2020. Available online: https://www.femteconline.org/NEWS/0075-balneology-immunology.pdf (accessed on 20 October 2022).
- Strauss-Blasche, G.; Gnad, E.; Ekmekcioglu, C.; Hladschik, B.; Marktl, W. Combined inpatient rehabilitation and spa therapy for breast cancer patients: Effects on quality of life and CA 15-3. Cancer Nurs. 2005, 28, 390–398.
- Vareka, I.; Stejskal, D.; Varekova, R.; Burianova, K.; Hnatek, J. Changes in Clusterin Serum Concentration Levels in Oncologic Patients During the Course Of Spa Therapy—A Pilot Study. Biomed. Pap. 2009, 153, 117–120.
- Kwiatkowski, F.; Mouret-Reynier, M.; Duclos, M.; Leger-Enreille, A.; Bridon, F.; Hahn, T.; Van Praagh-Doreau, I.; Travade, A.; Gironde, M.; Bézy, O.; et al. Long term improved quality of life by a 2-week group physical and educational intervention shortly after breast cancer chemotherapy completion. Results of the ‘Programme of Accompanying women after breast Cancer treatment completion in Thermal resorts’ (PACThe) randomised clinical trial of 251 patients. Eur. J. Cancer 2013, 49, 1530–1538.
- Kwiatkowski, F.; Mouret-Reynier, M.-A.; Duclos, M.; Bridon, F.; Hanh, T.; Van Praagh-Doreau, I.; Travade, A.; Vasson, M.-P.; Jouvency, S.; Roques, C.; et al. Long-term improvement of breast cancer survivors’ quality of life by a 2-week group physical and educational intervention: 5-year update of the ‘PACThe’ trial. Br. J. Cancer 2017, 116, 1389–1393.
- Yang, H.-L.; Chen, X.-P.; Lee, K.-C.; Fang, F.-F.; Chao, Y.-F. The Effects of Warm-Water Footbath on Relieving Fatigue and Insomnia of the Gynecologic Cancer Patients on Chemotherapy. Cancer Nurs. 2010, 33, 454–460.
- Cantarero-Villanueva, I.; Fernández-Lao, C.; Caro-Morán, E.; Morillas-Ruiz, J.; Castillo, N.G.; Rodriguez, L.D.; Arroyo-Morales, M. Aquatic exercise in a chest-high pool for hormone therapy-induced arthralgia in breast cancer survivors: A pragmatic controlled trial. Clin. Rehabil. 2012, 27, 123–132.
- Cantarero-Villanueva, I.; Fernández-Lao, C.; Cuesta-Vargas, A.I.; Del Moral-Avila, R.; Fernández-De-Las-Peñas, C.; Arroyo-Morales, M. The Effectiveness of a Deep Water Aquatic Exercise Program in Cancer-Related Fatigue in Breast Cancer Survivors: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2013, 94, 221–230.
- Mourgues, C.; Gerbaud, L.; Leger, S.; Auclair, C.; Peyrol, F.; Blanquet, M.; Kwiatkowski, F.; Leger-Enreille, A.; Bignon, Y.-J. Positive and cost-effectiveness effect of spa therapy on the resumption of occupational and non-occupational activities in women in breast cancer remission: A French multicentre randomised controlled trial. Eur. J. Oncol. Nurs. 2014, 18, 505–511.
- Reger, M.; Kutschan, S.; Freuding, M.; Schmidt, T.; Josfeld, L.; Huebner, J. Water therapies (hydrotherapy, balneotherapy or aqua therapy) for patients with cancer: A systematic review. J. Cancer Res. Clin. Oncol. 2022, 148, 1277–1297.
- Matceyevsky, D.; Hahoshen, N.Y.; Vexler, A.; Noam, A.; Khafif, A.; Ben-Yosef, R. Assessing the effectiveness of Dead Sea products as prophylactic agents for acute radiochemotherapy-induced skin and mucosal toxicity in patients with head and neck cancers: A phase 2 study. Isr Med. Assoc. J. IMAJ 2007, 9, 439–442.
- De Andrade, S.C.; de Carvalho, R.F.P.P.; Soares, A.S.; Freitas, R.P.D.A.; Guerra, L.M.D.M.; Vilar, M.J. Thalassotherapy for fibromyalgia: A randomized controlled trial comparing aquatic exercises in sea water and water pool. Rheumatol. Int. 2008, 29, 147–152.
- Chennaoui, M.; Gomez-Merino, D.; Van Beers, P.; Guillard, M.; Drogou, C.; Lagarde, D.; Bougard, C. Benefits of Thalassotherapy with Sleep Management on Mood States and Well-being, and Cognitive and Physical Capacities in Healthy Workers. J. Sleep Disord. Ther. 2018, 7, 5.
- Kim, N.-I.; Kim, S.-J.; Jang, J.-H.; Shin, W.-S.; Eum, H.-J.; Kim, B.; Choi, A.-R.; Lee, S.-S. Changes in Fatigue Recovery and Muscle Damage Enzymes after Deep-Sea Water Thalassotherapy. Appl. Sci. 2020, 10, 8383.
- Blain, H.; Bernard, P.L.; Canovas, G.; Raffort, N.; Desfour, H.; Soriteau, L.; Noguès, M.; Camuzat, T.; Mercier, J.; Dupeyron, A.; et al. Combining balneotherapy and health promotion to promote active and healthy ageing: The Balaruc-MACVIA-LR® approach. Aging Clin. Exp. Res. 2016, 28, 1061–1065.
- Greenlee, H.; DuPont-Reyes, M.J.; Rn, L.G.B.; Carlson, L.E.; Cohen, M.R.; Deng, G.; Johnson, J.A.; Mumber, M.; Seely, D.; Zick, S.M.; et al. Clinical practice guidelines on the evidence-based use of integrative therapies during and after breast cancer treatment. CA Cancer J. Clin. 2017, 67, 194–232.
- Turner, R.R.; Steed, L.; Quirk, H.; Greasley, R.U.; Saxton, J.M.; Taylor, S.J.; Rosario, D.J.; A Thaha, M.; Bourke, L. Interventions for promoting habitual exercise in people living with and beyond cancer. Cochrane Database Syst. Rev. 2018, 9, CD010192.
- Lyman, G.H.; Greenlee, H.; Bohlke, K.; Bao, T.; DeMichele, A.M.; Deng, G.E.; Fouladbakhsh, J.M.; Gil, B.; Hershman, D.L.; Mansfield, S.; et al. Integrative Therapies During and After Breast Cancer Treatment: ASCO Endorsement of the SIO Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 2647–2655.
- Grant, S.J.; Hunter, J.; Seely, D.; Balneaves, L.G.; Rossi, E.; Bao, T. Integrative Oncology: International Perspectives. Integr. Cancer Ther. 2019, 18, 1534735418823266.
- Ruiz Vozmediano, J. Influence of Diet, Physical Exercise and Mindfulness in Survivors of Stage IIa-IIb Breast Cancer. Ph.D. Thesis, Universidad de Granada, Granada, Spain, 2020. (In Spanish).
- Zhevago, N.A.; Zimin, A.A.; Glazanova, T.V.; Davydova, N.I.; Bychkova, N.V.; Chubukina, Z.V.; Buinyakova, A.I.; Ballyuzek, M.F.; Samoilova, K.A. Polychromatic light (480–3400 nm) similar to the terrestrial solar spectrum without its UV component in post-surgical immunorehabilitation of breast cancer patients. J. Photochem. Photobiol. B Biol. 2017, 166, 44–51.
- Ray, H.; Jakubec, S.L. Nature-based experiences and health of cancer survivors. Complement. Ther. Clin. Pract. 2014, 20, 188–192.
- Liamputtong, P.; Suwankhong, D. Therapeutic landscapes and living with breast cancer: The lived experiences of Thai women. Soc. Sci. Med. 2015, 128, 263–271.
More
