Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Lei Wang.
Prior methods of patient care have changed in recent years due to the availability of minimally invasive surgical platforms for endovascular interventions. These platforms have demonstrated the ability to improve patients’ vascular intervention outcomes, and global morbidities and mortalities from vascular disease are decreasing.
- robot-assisted vascular interventions
- haptic feedback
- control strategies
1. Introduction
The causative factors of atherosclerotic plaques within the human body are not fully understood. However, these plaques’ appearance, development, spread, and subsequent effects within the body have been studied extensively [1]. For instance, coronary artery disease (CAD) develops when atherosclerotic plaques (fat, calcium, and inflammatory cells) accumulate within the coronary arteries, resulting in the thickening of arterial walls and the obstruction of blood flow to the heart muscle [2]. Besides, these plaques manifest in the blood vessels located in the upper and lower extremities. Early symptoms of CAD frequently include pain in the shoulders, arms, or chest; numbness; myocardial infarction; and even sudden death [3]. As a result, CAD significantly contributes to global disease burden, mortality, and rising hospitalization costs [4].
Over time, open surgery, such as arterial bypass surgery, was performed to fix blocked coronary vessels to reroute blood flow to the heart. This treatment approach necessitates significantly large incisions in the patients’ chests, and it is commonly characterized by longer recovery time, risk of bleeding, potential infections at the incision site, and bad cosmesis [5]. Alternative therapies were required to treat vascular diseases because of the drawbacks of arterial bypass surgery. The development of interventional cardiology and the success of emerging technologies, however, were key factors in how vascular disease treatment has changed. As illustrated in Figure 1, the pioneering discovery of cerebral angiography by Egas Moniz in 1927 set the tone for today’s less invasive procedures [6]. Since then, different technological developments have been made to improve the diagnosis, treatment modality, and outcomes of minimally invasive procedures. At present, open-heart surgeries have been gradually phased out in favor of less invasive endovascular procedures that rebuild blood-flow pathways using endovascular tools [7]. This has become an effective treatment method for CAD, with patients benefiting from many of its advantages, including shortened recovery time and reduced perioperative risks [8].
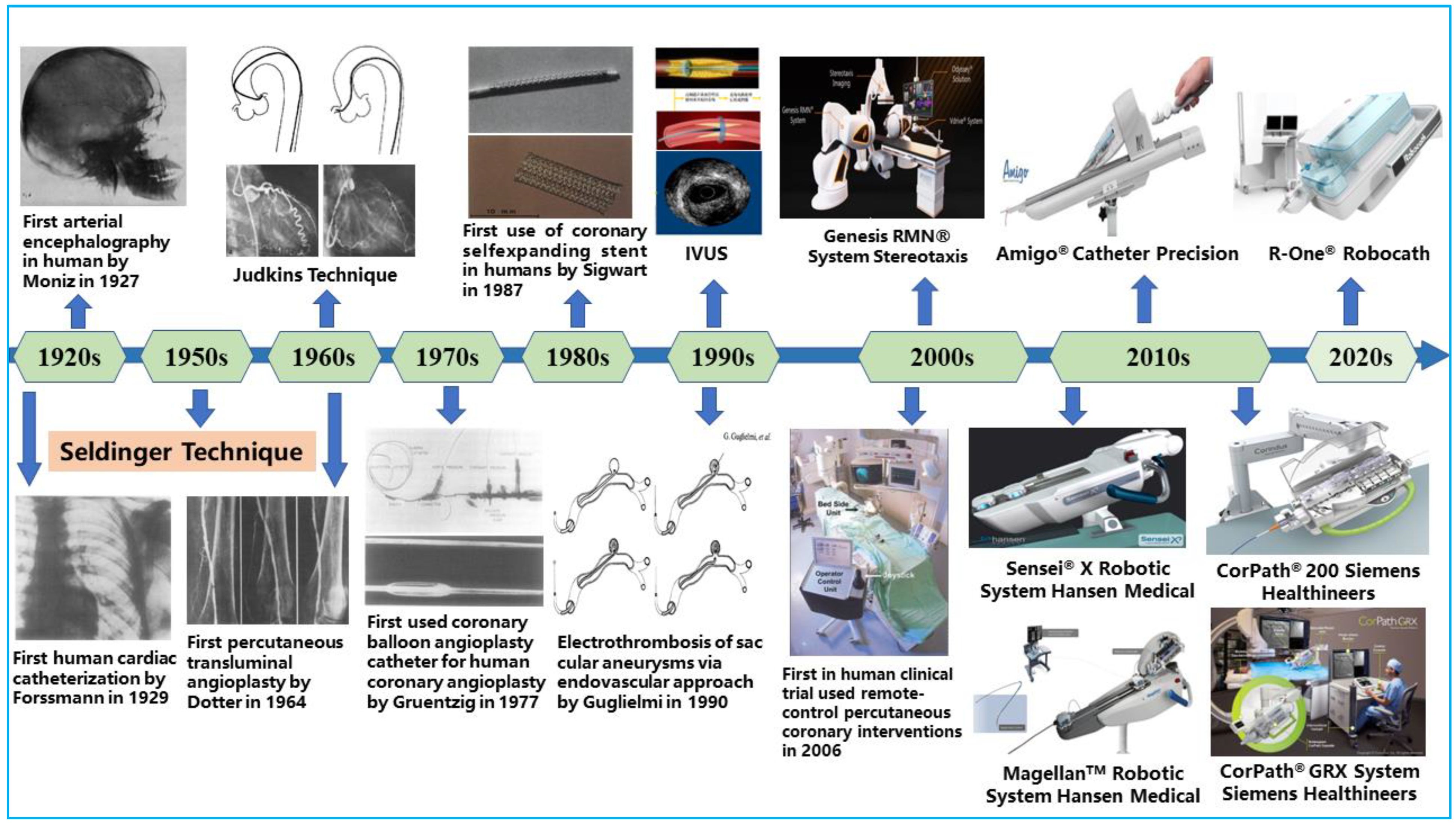
Figure 1.
Key milestones in the field of endovascular interventional therapy.
Skilled interventional cardiologists perform PCI procedures by navigating flexible endovascular tools such as guidewires, catheters, and stents from a peripheral entry port to a target site in the coronary arteries using their motor, cognitive, and procedural skills [9]. In addition, fluoroscopic-based systems are integrated within the cath labs to provide a field of view during procedures, localize the size of lesions, and guide the catheter’s axial and rotary navigation while preventing damage to the blood vessels. With these approaches, enhanced patient outcomes are achieved compared to arterial bypass surgery, but at a cost to the interventionalist’s long-term health [10]. Primary steps to safeguard the interventionalist’s health from scatter-radiation risks during procedures such as the adorning of radiation shielding were mostly operator-dependent and without substantial effectiveness [11]. In addition, an increase in physical discomfort and orthopedic injuries were prevalent amongst the interventionalists, as reported in several studies [12,13][12][13]. The conventional procedures required interventionalists to have high angiographic precision for stent placement. However, there were occurrences of geographic misses, imprecise stent placement, and miscalculated stent length largely due to visual estimation by the interventionalist [14].
The inherent concerns associated with conventional endovascular procedures continue to limit the widespread acceptance of less invasive therapies. A recent way forward was found through the introduction of robotic systems for improved procedures and patient outcomes [15]. Based on this, several studies have shown that robot-assisted interventions can overcome the drawbacks of conventional vascular procedures [16,17,18][16][17][18]. For instance, it helps physicians to improve navigation accuracy and enhance stability and precision during catheterization, and is capable of eliminating imprecise navigation due to the operator’s hand tremors, thus helping to minimize intraluminal vessel damage. More importantly, it allows the interventionalist to operate from a safe distance, thereby minimizing the operator’s exposure to scattered radiation while still maintaining a substantial field of view during procedures [19]. This proof of concept and early-stage demonstration of robotic-system feasibility for endovascular interventions has accelerated the development of several robotic-system prototypes at the commercial scale over the last two decades [20,21,22][20][21][22]. The use of surgical robots for different interventions has gradually increased in the operating room due to advantages such as operation speed, navigation precision, dexterity, and action reproducibility when compared with expert human performance [23]. Similarly, within the research domain, research-based prototypes of robotic systems for PCI procedures have been developed and are being scaled up for commercial viability [24,25,26,27,28][24][25][26][27][28]. Furthermore, the safety, feasibility, and clinical adoption of existing robotic systems for neurovascular interventions, cardiovascular interventions, peripheral vascular interventions, and electrophysiological interventions have been reported in several studies [29,30,31,32,33][29][30][31][32][33]. These interventional domains formed the bases for the advocacy of the adoption of robot-assisted endovascular interventions.
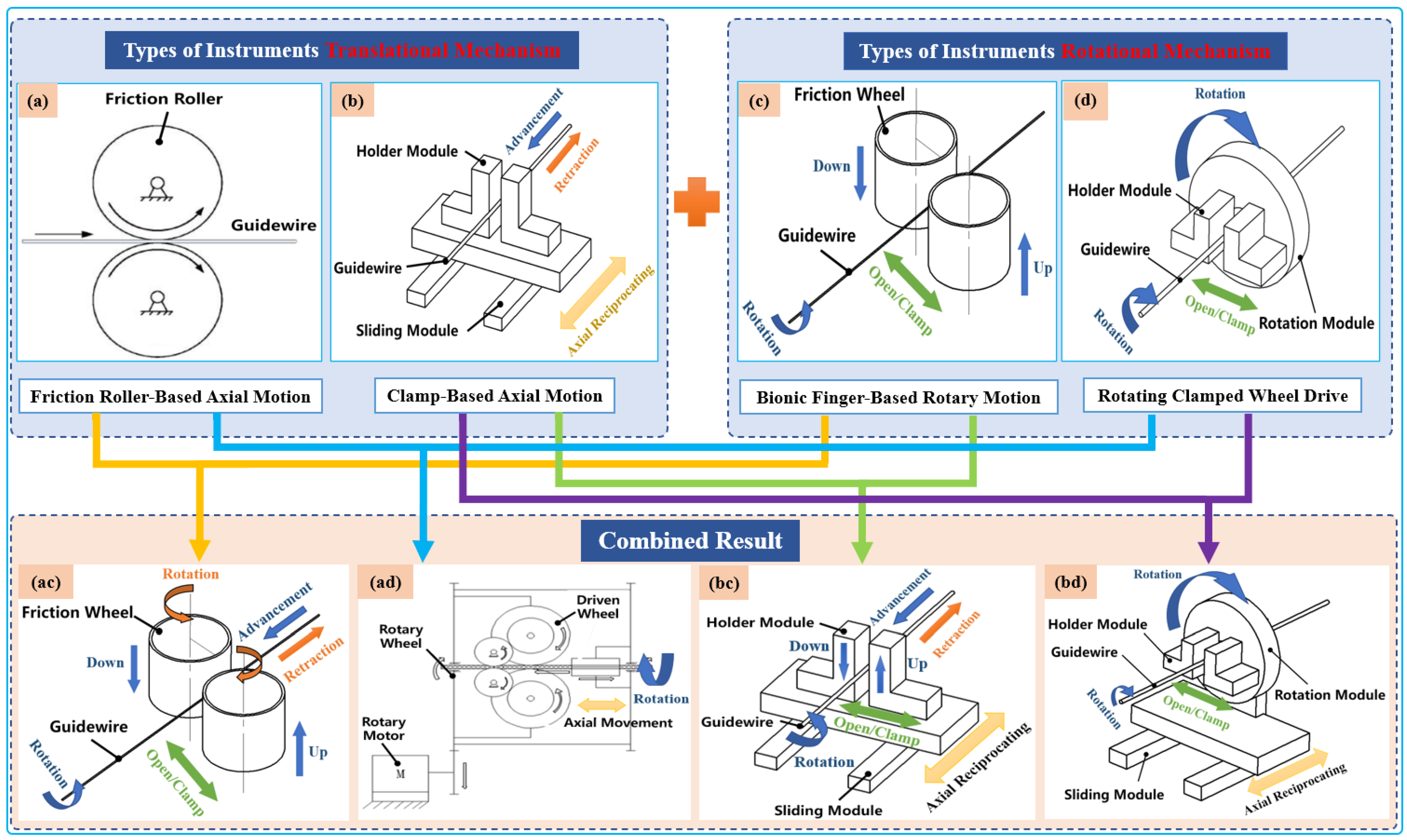
 Typically, two types of axial-drive mechanism are utilized in VIR for highly precise linear motion, which is then combined with two rotating mechanisms. Both combinations can achieve four different modes of axial and rotary mechanisms that can generate simplified 2-DOF tool motion, as illustrated in Figure 3. Currently, VIRs mostly use an arrangement of one of these axial and rotary mechanism to drive the guidewires or catheters intuitively during procedures [40,41][40][41]. Therefore, an overview of these modes is presented below.
Typically, two types of axial-drive mechanism are utilized in VIR for highly precise linear motion, which is then combined with two rotating mechanisms. Both combinations can achieve four different modes of axial and rotary mechanisms that can generate simplified 2-DOF tool motion, as illustrated in Figure 3. Currently, VIRs mostly use an arrangement of one of these axial and rotary mechanism to drive the guidewires or catheters intuitively during procedures [40,41][40][41]. Therefore, an overview of these modes is presented below.
2. Key Technologies and Application Areas of Vascular Interventional Robots
Recent advances in the fields of robotics, sensors control, computer vision, and artificial intelligence have fueled rapid growth in the area of medical robotics [34,35,36][34][35][36]. Over the last few decades, several types of endovascular robots have been developed. Typically, VIR combines sensing, actuation, and tool-clamping mechanisms with a wireless communication protocol, control system, and user interface for robust endovascular procedures. Such procedures require efficient collaboration between the surgeon and the robot to achieve safe, precise, and dexterous tool movement within the patients’ blood vessel. In addition, control and safety strategies are implemented within the master–slave platform coupled with image-based guidance systems to minimize operative risks during procedures. Furthermore, the tool–vessel contact force and haptic feedback are essential for closed-loop control modeling and potential autonomous navigation based on increased machine awareness and operation safety during catheterization [37,38][37][38]. These essential areas for safe robot-assisted vascular interventions are discussed elaborately in the subsequent sections.2.1. Driving Mechanisms and Teleoperation Setups
2.1.1. Classification by Driving Mechanisms
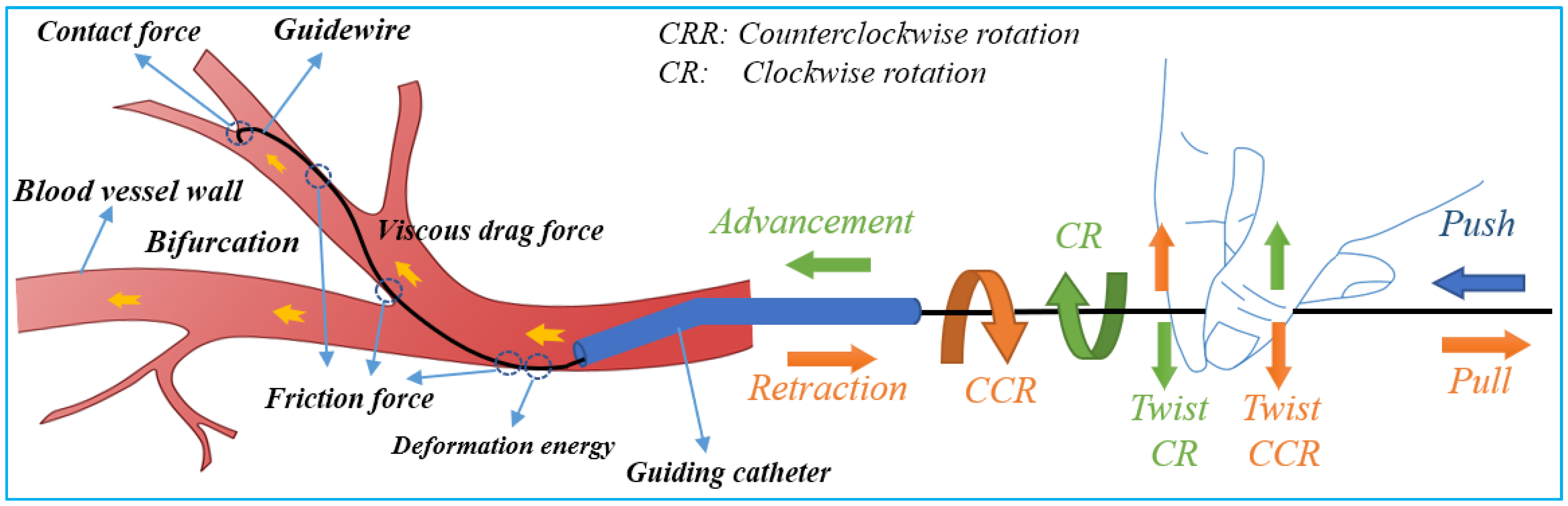
Safe navigation of flexible endovascular tools through blood-vessel paths for stent and balloon delivery requires that surgeon uses their forefinger and thumb skillfully [39]. These intelligent-hand defter procedures requires stimuli interplay of kneading the endovascular tools to manipulate the thin, long, and flexible tools back and forth along blood vessels. When a bifurcation is encountered, the two fingers are rubbed up and down in relation to each other to change the direction of the guidewire tip in order to pass through the bifurcation. The schematic diagram of guidewire manipulations and force analysis by the interventionalist’s thumb and index fingers is shown in Figure 2. Based on the manipulation modes illustrated in Figure 2, it can be seen that the motion of the robot requires at least three actions—clamping, translation, and rotation for two degrees of freedom (2-DOF) of endovascular tool motion (axial and rotary) within the blood vessels.
Figure 2.
Schematic of guidewire manipulations and force analysis on the surgeon’s fingers.

Figure 3. Classification schematic diagram of instruments’ driving mechanisms. Types of translational instrument mechanisms: (a) friction roller rotating along its axis to translate guidewires; (b) holder module clamp guidewire axial reciprocating motion on the sliding module. Types of rotational instrument mechanisms: (c) two bionic clamp fingers move linearly in the opposite direction to rub the guidewires; (d) holder module clamp guidewires rotated on the rotation module. Combined result: (ac), (ad), (bc), and (bd) are the result of permutation and a combination of translational instrument mechanisms with rotational mechanisms to realize the simultaneous advancement and rotation of guidewires.
- (1)
-
Translational Tool Mechanisms
- (2)
-
Types of Rotational Instrument Mechanisms
2.1.2. Teleoperation Setup
In order to reduce occupational hazards such as radiation exposure and orthopedic injuries that surgeons experience during endovascular procedures, VIRs have been designed with capabilities for remote manipulation. This usually involves the use of bedside instrument tool-driving mechanisms (slave device) and a control interface (master device) that the operator handles at the cockpit station. For ease of usage at the control station, interventionalists often visualize the surgical scene on multiple display units and access the master device for tool manipulation. This setup serves its purpose by protecting the surgeon from scattered radiation and grants them ergonomic comfort to cannulate the patients’ blood vessel under imaging guidance [47]. At present, the design of the master–slave robot platform includes isomorphic and non-isomorphic teleoperation setups, as highlighted in Table 1 and described below.- (1)
-
Isomorphic setup: An isomorphic teleoperation involves using more ergonomic master interfaces that allow surgeons to replicate their natural hand-movement patterns during interventions. In this setup, the master-and-slave systems have similar structural and functional designs. Thus, the actions commands issued on the master side are homogenously replicated in the slave-side device. This makes slave devices exhibit interventionalists’ hand-and-finger dexterity for fine motor-based tool manipulation. Isomorphic setups are new in the endovascular intervention domains. However, recent studies have shown that it can reduce surgeons’ learning curve since they can directly utilize their natural catheterization skills. The isomorphic design by Thakur et al. [25] directly utilizes a real input catheter as the master device and a sensor to record the catheter’s motion while the slave device replicates the master motion to drive a catheter inside the vessel. Similarly, Payne et al. [48] developed a novel master–slave force-feedback system that conforms to a doctor’s natural operating habits and ergonomics. The interface of the same configuration is in line with the intuitive operation of doctors, which is easier to understand and learn. More and more isomorphic platforms have been developed and utilized in recent studies [49,50,51][49][50][51].
- (2)
-
Non-isomorphic: Non-isomorphic teleoperation design is the earliest and most common approach utilized in robot-assisted minimally invasive interventions. In this mode, a significant difference exists in between the structural design of master-and-slave devices in robotic-platform structural design. Specifically, the robotic setup has master-and-slave control interfaces with a unique design and tool-handling schemes. Currently, most of the commercial robotic systems used for endovascular interventions are generally non-isomorphic. For instance, the control interfaces in CorPath® GRX and CorPath® 200 robotic catheter systems are based on joysticks and touch screens [52]. The typical designs of the CorPath interfaces allows surgeon to manipulate endovascular tools like guidewires with one hand and operate other tools such as the balloon/stent catheter with the other hand. Similarly, in the Amigo® system (Catheter Precision, Inc., Ledgewood, NJ, USA), another major commercial interventional robot used for electrophysiological interventions, the master device is designed as a wireless remote controller for catheter manipulation. The system is able to reproduce linear catheter motions, rotary motion, and tip deflection all issued by the appropriate buttons with one hand on the master device [53]. Although this controller system has an intuitive input method, the design and form are essentially different from the slave robotic platform. Relatedly, some other non-isomorphic setups involve the use of commercial 3-DOF haptic devices as the master-side platform. Typically, Ma et al. [54] and Shen et al. [45] selected Omega (Force Dimension, Nyon, Switzerland), a parallel manipulator capable of producing force feedback to the operator, as the master interface. The commercial controllers are generally adaptable to existing robotic systems. However, customizing them for tool-delivery mechanisms is sometimes difficult.
2.2. Guidance Systems and Robotic Control Scheme
2.2.1. Image-Based Guidance Systems
The exact navigation of endovascular tools within the blood vessels is a key aspect of minimally invasive interventions. During these procedures, the surgeon aims to maintain a continuous mental grasp of the endovascular tool’s actual position in order to steer the guidewire or catheter safely within the vasculature. However, to achieve this, image-based guidance systems are designed to complement VIRs. These systems visualize the catheter’s position non-invasively, localize coronary lesions, and help to minimize the occurrence of ruptures during procedures. For endovascular interventions, a number of catheter-guidance technologies have been developed and adopted in the cath lab. Broadly speaking, these can be divided into extravascular imaging-system modalities, such as digital subtraction angiography (DSA), computed tomography (CT), magnetic resonance (MR), and ultrasound (US), and intravascular imaging modalities, such as intravascular ultrasound (IVUS) and optical coherence tomography (OCT). Currently, DSA is the imaging technique used most frequently by interventionalists because of its higher spatial and temporal resolution. With the DSA system, the physician can choose the interventional path or pinpoint the lesions’ size and distribution based on anatomical knowledge and real-time 2D angiography and fluoroscropy sequences of vessel imaging. The 2D-imaging technique is quick and clear enough to meet the needs of real-time intraoperative vascular imaging; however, it lacks 3D spatial information [55]. In contrast, 3D vascular images can be reconstructed using CTA. Typically, to compensate for the lack of 3D spatial information in the 2D image during operation, a preoperative vascular model is constructed prior to the procedure and registered with the 2D image taken in real-time during procedures. This offers an intuitive visual reference for precisely tracking the placement of surgical tools inside vessels for diagnosis and treatment. However, the disadvantages of 3D CTA include the low signal-to-noise ratio, large radiation dose, insufficient real-time performance, and presence of artifacts [56]. Compared to fluoroscopic imaging, MRI systems can produce both 2D and 3D images (MRA—magnetic resonance angiography), have high contrast to soft tissue, and pose no radiation risk. However, due to the presence of breathing and heartbeat movements, as well as anatomical factors like vascular torque and venous structure overlap, the image quality depreciates, resulting in artifacts and other defects present within the images. In addition, the patient is in a small, closed-loop scanner during MRA, which presents a significant challenge to the surgical robot’s structural design and magnetic compatibility [57]. US imaging technology can be utilized to assess the location, size, and shape of tissues and organs as well as the extent of lesions. It also has a significant effect on soft tissues and can provide depth information. It can be used in addition to 2D fluoroscopy images and is non-radiative, portable, and easy to use. However, its use in vascular interventional surgery is constrained by the inability to accurately visualize the catheter or guidewire’s spatial pose, which is a drawback [58]. Recent years have seen a rapid development of a number of intravascular imaging techniques that can navigate through smaller vascular lumen prior to and post-stent implantation and can be used to evaluate the plaque coverage, stent placement, and expansion degree. IVUS and OCT are the intravascular imaging techniques that have received the most research to date. IVUS creates images using high-frequency ultrasound to assess the degree of vascular stenosis and identify bifurcations and calcified lesions, among other things. These images reflect the layered structure of vascular tissue. However, the primary drawback of IVUS is its low resolution, which makes it challenging to determine the fibrous cap and hyperechoic plaques’ exact thicknesses. The use of IVUS in small vessels and severely stenotic vessels is also limited by the size of the ultrasound probe. In contrast, OCT can detect and categorize plaques more precisely than IVUS because of its higher spatial resolution and imaging-acquisition speed, which can be up to 10 and 40 times higher, respectively. Its disadvantage is that when blood flow is present, it has lower imaging quality and tissue penetration [59]. However, by combining the penetration of IVUS with the high resolution of OCT, many researchers have developed a hybrid IVUS–OCT probe to improve the accuracy of intravascular imaging and navigation [60]. Beyond this, some other studies have fused IVUS/OCT images and angiographic images to create 3D vessel reconstruction and to determine the position and direction of the catheter, which could open up alternative intraoperative 3D navigation [61].2.2.2. Robotic-Control Scheme
In robot-assisted endovascular interventions, the master–slave teleoperated system utilizes a control loop involving the human operator. Typically, the master controller deduces the surgeon’s actions and transfers corresponding input signals to the slave controller. The latter outputs appropriate control signals to the linear drive system for axial and rotational tool movements. However, in real systems, the slave robot’s linear drive mechanism experiences motion lag resulting in a slight deviation from the master’s input motion commands. This difference caused by tool–tissue friction, communication delay, nonlinear disturbances such as hysteresis and backlash, and other effects generally leading to inaccurate master–slave position trajectory and fluttering, which may cause tool drift and vascular perforation. Therefore, control systems are essential to minimize this effect and should have desirable characteristics such as high precision, fast response, tremor elimination, and surgical-safety early warning. Thus, several feedforward and feedback control-system implementations exist for different master–slave robotic systems. Open-loop control using position-control mode utilizes a feedforward controller, which aims to provide precise positioning without reliance on the slave robot’s output feedback. This is often a common control strategy [62]. However, feedforward systems could be simplistic, inaccurate, and unreliable for motion-control tasks essential for robot-assisted catheterization. An example of the feedforward control systems for VIR include that in Thakur et al. [25], where the authors utilized a constant scaling factor for master–slave motion mapping. Although this method could improve the master–slave position accuracy, it is a non-adaptive approach that could often require retuning and the occurrence of errors for unbounded intervals. Whereas the former study was based on constant scaling, Feng et al. [63] utilized an adaptive motion-scaling method. This was deployed into the master–slave device for adaptive tool navigation during robot-assisted vascular interventions. The authors evaluated the master–slave position deviation and introduced different scaling factors for proportional, reduced, or magnified input-to-output commands for different segments of a catheterization stroke. However, the practicability of the techniques in real-time systems poses concern such that closed-loop control systems have received increased attention for position tracking and error compensation to improve the accuracy of the catheter/guidewire insertion and navigation within the vasculature. This includes position control, force-based control, motion compensation, image-based navigation, and learning-based control schemes based on deep-learning and reinforcement-learning algorithms. These control methods have been applied to VIRs remarkably. The PID controller is the most commonly used control approach in VIRs and consists of tuning proportional, integral, and differential gains for smooth motion during robotic catheterization. Some configurations of PID-based controllers have been applied within this domain for master–slave position-error compensation. For example, in Ref. [44], the authors utilized the PID controller within their master–slave platform for control of the endovascular tool’s axial and rotational movements during robot-assisted peripheral vascular intervention. The PID control gains were utilized to determine matching step values for the linear drive actuator to obtain uniform input–output position commands. Overall, the controller compensated for the initial position error; however, the final error was around 0.5 mm. Similarly, Sankaran et al. [24] developed a cascade controller, which integrated an adaptive input shaper and the PID controller to achieve closer master–slave position tracking during robot-assisted catheterization. The study used guidewire resistance force as a measure of proximal feedback to enhance patient safety during catheterization. However, conventional PID controllers have some inherent limitations, including the occurrence of noise in the derivative gains, poor real-time performance, and consistency required for smooth catheterization during robotic interventions. Based on this, several research groups have proposed cascaded configurations of fuzzy–PID controllers employing fuzzy rules to fine-tune the linear parameters of the PID control gains dynamically. For instance, Song et al. [64] proposed the position control of a master–slave robot using intelligent fuzzy–PID controllers capable of online PID control gains and fuzzy-rule tuning. In addition, Yu et al. [50] developed a dual fuzzy–PID controller for online control-parameter tuning and interference removal in a VIR. Furthermore, Guo et al. [65] implemented fuzzy–PID controllers within a slave robotic device to improve the slave robot’s position-tracking capability with the issued master command. Compared with the conventional PID controllers, fuzzy–PID does not require an accurate methodical model and can better deal with time-varying, non-linear hysteresis problems, with good robustness and fast response time. However, the control rules are non-adaptive and require more time to be appropriately designed [66]. Besides classical controllers, Wang et al. [28] designed an adaptive sliding-mode controller for a master–slave system to resolve the nonlinear and uncertain disturbances that the catheter/guidewire encounters within a linear drive system, thereby reducing the deviation from the input motion command and improving the response speed and accuracy of the control system. The controller had a better performance than PID, with a final error between 0.07 and 0.3 mm. In contrast, Omisore et al. [67] proposed and developed an adaptive neuro-fuzzy control system in a 2-DOF robotic catheter system for backlash compensation and force control using the robots’ kinematic parameters. The in-vitro experiments validated the neural network model’s aptness for improving position-tracking error within the slave robot; hence, a final error of 0.4 mm was obtained. Recently, Zhou et al. [68] adopted an auto-disturbance rejection-control approach for a VIR. The model’s working principle hinges on tuning four subcomponents to control the target displacement and improves the real-time position-tracking accuracy of an endovascular tool. In comparison, the control accuracy and response speed of the control strategy highlighted above were much better than the conventional PID control method. Overall, these models yielded good position control, and they could better handle the input–output dynamics in the master–slave setup. Furthermore, the studies show that the model offered fewer errors compared with PID-based controllers. Despite the above-mentioned merits, machine-learning and artificial-intelligence algorithms are being utilized in newer VIR control models. These AI-based control models can achieve better tool catheterization and human–machine collaborative control. For instance, Ma et al. implemented a multi-layer neural-network model to tune PID parameters effectively and to improve the accuracy of the slave robot’s axial displacement. The study compared the MLP-tuned PID controller with conventional PID, and the result indicates that the neural-network-tuned controller had a better performance than the traditional PID control system [69]. Similarly, Wu et al. [70] utilized the long short-term memory network (LSTM) to model the hysteretic effects of a unidirectional robotic catheter and to track the position accuracy of its tip under different twist angles using the catheter’s kinematic parameters. Recently, Omisore et al. [66] proposed a deep reinforcement-learning model that could adaptively tune PID control gains for responsive tool tracking during robot-assisted PCI. The model evaluated via in-silico experiments achieved high tool-position accuracy with an RMS error of 0.003 mm. An advanced strategy published by Kweon et al. [71] shows that imitation or reinforcement learning can be directly designed for autonomous navigation of endovascular tools. In addition, Karstensen et al. [72] adopted deep deterministic policy gradients with hindsight experience replay for a learning-based control of guidewire navigation in a robot-assisted peripheral vascular-intervention study. The reinforcement-learning-based model was not reliant on human demonstration examples and had a 100% success rate for simulation-based studies. However, a lower precision was reported for the ex vivo study. Generally, in vivo applications of learning-based control models are still lacking. So far, most studies have focused on master–slave control accuracy and safety, and the emphasis on patient safety and excellent control modes has led to the evolution of different control models with their respective advantages. However, at present, there is no widely accepted control method approved as the standard for position accuracy in VIR. Each control method is analyzed based on its strengths and limitations; however, in the future, the realization of a consensus could be possible. In conclusion, a list of contemporary robotic systems developed and commercialized for endovascular interventions is presented in Table 1. These vascular robotic systems are categorized based on the key technologies discussed above with application areas covering endovascular interventions.Table 1.Summary of related robotic systems for vascular intervention.Group Driving Mechanisms
(Translation/Rotation)Teleoperation Setup Control Scheme Perception/Feedback Guidance Systems Application Areas CorPath® 200&GRX
[73,74,75][73][74][75]Friction roller-based/Rotating clamped wheel Non-Isom. Position and velocity Obstacle feedback DSA PCI
PVI
NVIMagellanTM
[42,76][42][76]Friction roller-based/Friction wheel-based rotation Non-Isom. Position and velocity Haptic DSA/CT PVI Guo et al.
[26,77][26][77]Clamp-based/Rotating clamped wheel Non-Isom./Isom. Position and force Haptic/Proximal force DSA PCI Wang et al.
[28,78][28][78]Clamp-based/Rotating clamped wheel Isom. Position and force Haptic/Proximal force DSA PCI Wang et al.
[40,79][40][79]Friction roller-based/Bionic finger-based rotary Non-Isom./Isom. Position and velocity N/A DSA NVI Wang et al.
[44,80][44][80]Clamp-based/Rotating clamped wheel Non-Isom. Position and velocity N/A DSA PCI
PVICha et al.
[27,81][27][81]Friction roller-based/Rotating clamped wheel Non-Isom. Position and force Haptic/Proximal force DSA PCI Choi et al.
[82]Friction roller-based/Bionic finger-based rotary Non-Isom. Position and velocity N/A DSA PCI Yang et al.
[48,83][48][83]Friction roller-based/Rotating clamped wheel Isom. Position and force Haptic/Distal force MRI PCI Tanimoto et al.
[84]Friction roller-based/Rotating clamped wheel Isom. Position and force Haptic/Distal and Proximal force CT PCI Omisore et al.
[67,85][67][85]Clamp-based/Rotating clamped wheel Isom. Position and force Haptic/Proximal force DSA PCI Bian et al.
[41,43][41][43]Friction roller-based/Bionic finger-based rotary Isom. Position and velocity N/A N/A PCI Zhou et al.
[86]Clamp-based/Rotating clamped wheel Non-Isom. Position and force Proximal force N/A PCI Li et al. [87] Clamp-based/Rotating clamped wheel N/A Position and velocity N/A IVUS-OCT PCI Langsch et al. [88] N/A N/A Position and velocity Proximal force US PCI Abbreviations: PCI—percutaneous coronary intervention; PVI—peripheral vascular intervention; NVI—neurovascular intervention; N/A—not applicable.2.3. Perception Systiems
During endovascular procedures, the operator should perceive the tactile feedback of the force with which the endovascular tool is steered within the vasculature during robotic catheterization. However, current VIRs are only able to detect clamping and proximally applied force. However, most existing VIRs are primarily limited by their inability to sense and provide feedback on the interactive tool–vessel contact force during procedures. The absence of tool–vessel contact force increases the possibilities of operative risks such as thrombosis and vascular perforation that could arise from reliance on visual feedback, poor hand–eye coordination, and its impact on the mental and cognitive load of the interventionalist during procedures [89]. To resolve this lack of force feedback, researchers are exploring different approaches to measure the distal and proximal force in VIRs. This will enhance surgeon’s telepresence and feedback on applied manipulation force during robotic procedures.2.3.1. Force Feedback
Proximal and distal force measurements are essential in VIR safety and enhanced operation strategy during procedures. Whereas the former is measured nearer to the operating end of the catheter via off-the-shelf force sensors, the latter is measured towards the farthest point along the catheter’s length and is of more concern to the interventionalist. The distal force measures the contact force between the tip of catheter and the vessel walls and requires a miniaturized sensor that should be embedded within the coronary catheter. Therefore, these sensors’ dimensions, resolution, biocompatibility, measurement range, and accuracy are critical characteristics [90]. Both vascular and electrophysiological interventions have applications of force-measuring catheters. In electrophysiological interventions, for instance, force-measuring catheters are used to determine the catheter’s electrode contact with the myocardium. This helps in preventing mounting excessive contact force when creating scars on abnormal heart tissues. This technology has attained commercialization, and some available products are the TactiCath® Catheter (Endosense SA, Geneva, Switzerland) and IntelliSense® System. The contact-force catheter of TactiCath is a steerable 7-F radiofrequency-ablation catheter that integrates a force sensor at the distal end and measures the contact force between the catheter tip and heart tissue [91]. To improve stability under dynamic conditions during vascular interventions, a force-measuring catheter usually integrates the strain gauge or pressure-sensitive rubber and encapsulates the active part of the catheter with biocompatibility. For example, Guo et al. [92] arranged the pressure-sensitive rubber-sensing units in the front-end array of ducts and encapsulated them to detect the forces of different nodes. Omisore [67], Payne [48], and Wei [93], amongst many, designed catheters with over-the-wire force sensing (i.e., using strain gauge) to measure intravascular contact forces. Although resistive and strain sensors have excellent linearity, hardware such as circuits and metal substrates are vulnerable to electromagnetic interference during surgery, and the size is too large to be suitable for narrow vascular cavities [94]. FBG sensors have the advantages of small size, no electromagnetic interference, and high sensitivity. Recently, researchers integrated FBG-based fiber-optic force transducers into the catheter to solve the problem of size and electromagnetic interference [95]. He et al. [96] developed a catheter integrating four FBGs for intravascular force measurement and temperature compensation. Although distal forces are important during electrophysiological ablation, the natural sense of touch felt by the surgeon is the proximal force in manipulating surgical instruments during traditional vascular interventions. The four intravascular force components constitute a complete proximal force: the viscous force of the blood, the collision force at the front end of the instrument, the friction force between the blood vessels, and the potential elastic force of the guidewire [20], as shown in Figure 4. The proximal-force measurement is usually carried out in a sensor-based manner, in which a high-precision and high-resolution force sensor is installed on the device-delivery mechanism to measure the contact force between the device and the blood vessel. However, there are also differences in the sensor installation and the force-measurement method. For instance, Yang et al. [97] developed a guidewire force-measuring mechanism based on the lever principle. The pressure sensor is installed on the propulsion finger, and the resistance is transmitted to the force-measuring mechanism through the guidewire. As the lever amplifies the signal, the force-measuring mechanism is unaffected by the disturbance in the transmission structure. Bao et al. [98] installed the force sensor on the clamping side of the guidewire, use the linear bearing to reduce the interference of friction on the guidewire resistance in the transmission process, and designed a multi-level safety-control strategy according to the force level to reduce the risk of operation. Similarly, Zhou et al. [86] and Wei et al. [93] use the force-measurement method of installing a sensor in the guidewire clamping part to measure and evaluate the resistance of the guidewire to improve the safety control of the robot. Sankaran et al. used the current of the drive motor to estimate the resistance of the guidewire and used the double-layer optimization method to calibrate [24]. The above sensor-based proximal force-measurement methods are most sensitive and generally able to measure the approximate value of resistance when the translational speed is low, the inclination angle is minimal, and the friction of the mechanism is very small [99]. However, during the robot’s operation, the resistance value obtained proximally is often inaccurate and susceptible to interference, mainly arising from weak resistance caused by either the frictional force in the actuator, the inertia force, or the presence of jerk in the linear drive system [100,101][100][101].In addition to sensor-based force-measurement methods, model-based force measurement is also available. The force of the device in the model is calculated and estimated by combining sensor boundary conditions, real-time imaging, vascular anatomy, and the device model [102]. Therefore, the mechanical model of the guidewire or catheter must be accurate, and the solution process must be in real time, accurate, and stable. There are many modeling methods: the continuum mechanics model, many-body dynamics model, differential geometry model, and particle-based model. Due to the low cost and low external interference, model-based methods have gradually become a new direction of force-feedback research [103]. However, the disadvantage is that the nonlinear integration of the dynamic model may be numerically unstable and computationally expensive, so it is not easy to realize.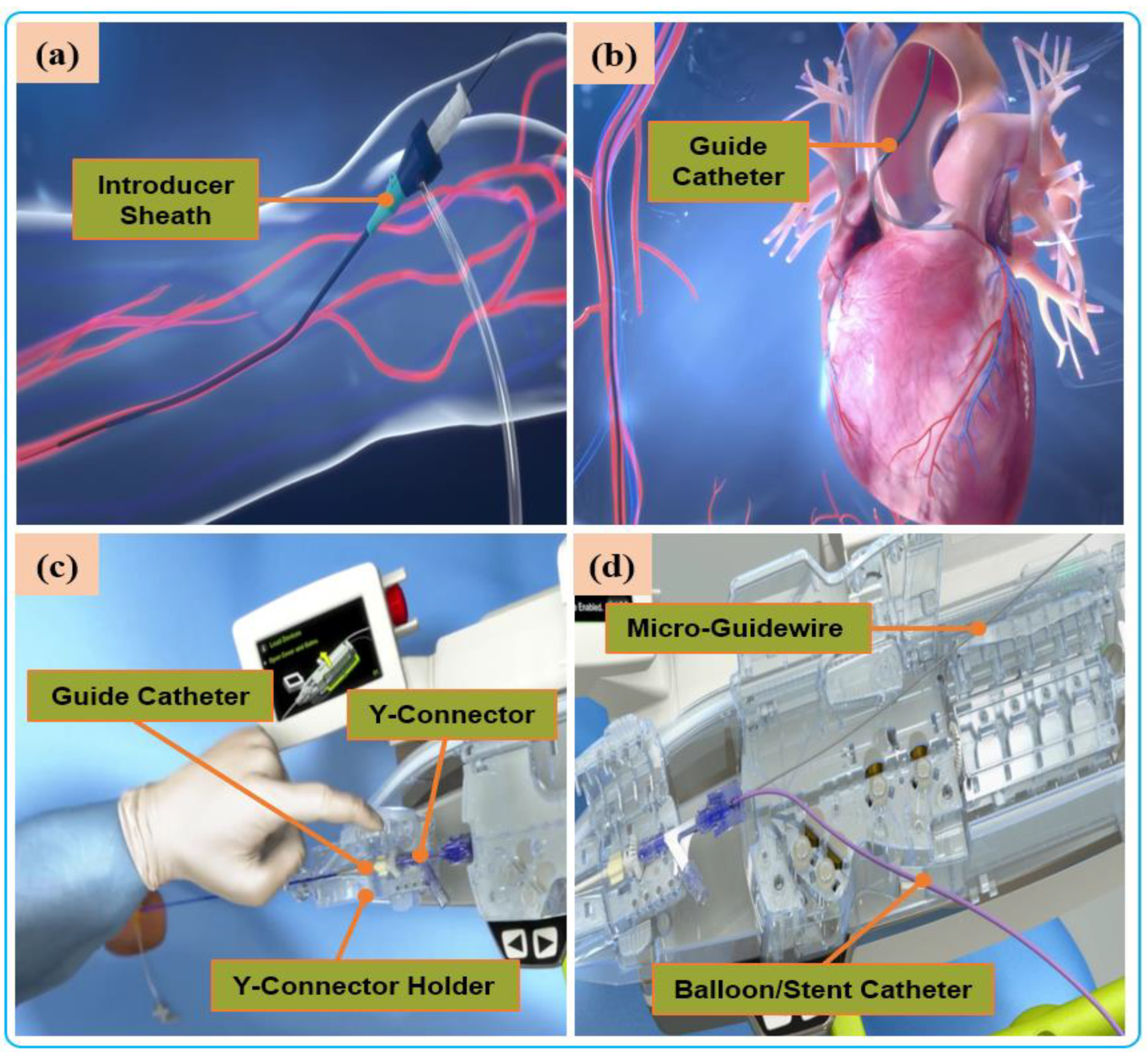 Figure 4. Manual procedure: (a) surgeon makes a small incision in the femoral/radial artery and insert an introducer sheath into the body to establish access for external instruments. (b) The guide catheter is hung on the coronary artery opening. (c) The guide catheter is attached to the Y connector and installed on the Y-connector holder to establish a stable track for device delivery. (d) A micro guidewire and balloon/stent catheter is loaded close the shell.
Figure 4. Manual procedure: (a) surgeon makes a small incision in the femoral/radial artery and insert an introducer sheath into the body to establish access for external instruments. (b) The guide catheter is hung on the coronary artery opening. (c) The guide catheter is attached to the Y connector and installed on the Y-connector holder to establish a stable track for device delivery. (d) A micro guidewire and balloon/stent catheter is loaded close the shell.2.3.2. Haptic Perception
Haptic perception is to transmit the feedback force between devices and vessels to the surgeon’s operation side through the control system. Through human–computer interaction, the interventionist can feel the resistant force of the endovascular tools, thus providing force perception and feedback, which can reproduce the haptic perception of vascular surgeons in traditional surgery and reduce the risk of surgery [104]. Amongst the commercially available master manipulators with force-feedback capacity, the Phantom Omni (Sensable Technologies, Wilmington, MA, USA) and Geomagic Touch X (3D Systems, Rock Hill, SC, USA) are the first to be used for catheter-insertion control and tactile feedback [105]. Their main structure is a series-control mechanism designed based on motor current to output torque and braking force. Another device, Omega Haptic Devices uses a Delta-based parallel-control structure. It is widely used in the master manipulator of medical robots due to flexible control, high spatial resolution, and sensitive force feedback [106]. However, these commercial robot-operated main hands have a much longer learning curve when it comes to surgical interventions that go against the surgeon’s traditional surgical skills [89]. Therefore, to better adapt to the clinical needs, researchers have conducted in-depth research on the intuitive master–slave isomorphic force-sensing control mechanism. Yan et al. [97] designed a master–slave isomorphic surgical robot. The master force sensing realized surgeons’ precise perception of the slave guidewire force through the interactive predictive control of the motor, torque sensor, and guidewire resistance. Guo et al. [26] proposed the haptic feedback technology based on magnetorheological fluid to measure the operator’s movements and provide haptic force. The in vitro experimental results showed that the haptic feedback based on catheter was helpful for improving intubation skills and reducing the cognitive workload of operators. Li et al. [107] used similar principles to develop a collaborative control platform based on magnetorheological fluid and hydrogel modeling that could replicate operator movements while providing haptic force feedback. Payne et al. [48] developed a “hands-on” master–slave control system that used voice-coil motors to provide force feedback to the operator. Through body-membrane experiments, it was verified that the contact force between the catheter and the tissue could be significantly reduced. Commercial master manipulators provide steady force feedback but are a challenge for doctors to learn their skills. However, a master control mechanism with an isomorphic master–slave limitedly supports surgeons’ surgical habits. Thus, some challenges such as unstable force feedback, high friction force, and inability to eliminate doctors’ hand tremor needs further study.2.4. Application Areas of Vascular Interventional Robots
VIRs are categorized based on their application areas. Robot-assisted vascular intervention can be divided to into four, which include robot-assisted percutaneous coronary intervention (R-PCI), robot-assisted peripheral vascular intervention (R-PVI), robot-assisted neurovascular intervention (R-NVI), and robot-assisted electrophysiological intervention (R-EPI). Three of these specialty domains (R-PCI, R-PVI, R-NVI) possess some similarities in the way the procedures are carried out except that the location of the blood vessels differs from one another, such as the heart coronary, the lower extremity, and the head region. Nevertheless, commercial robotic systems were initially designed as sub-specialty systems targeted at R-PVI or R-PCI. This includes the Hansen Magellan system for R-PVI and the CorPath robotic systems and R-One robotic System (Robocath Inc., Rouen, France) for R-PCI. However, effort has been made to showcase the ability of one of the systems to be adaptable for the three vascular procedures with or without minor modification. For example, the CorPath® GRX robotic system has been tested for R-PCI, R-PVI, and R-NVI procedures [73,74,75][73][74][75]. Hence, it can be classified as a multi-specialty vascular robotic system. R-PCI, R-PVI, and R-NVI are classified as endovascular interventions. These procedures use flexible tools such as catheters, guidewires, balloons, and stents, which are navigated to the site of the vessel lesion by VIR systems. On the other hand, robot-assisted electrophysiological procedures have distinct anatomical objectives and a procedural flow that is different from the abovementioned vascular procedures; therefore, electrophysiological-based interventional robots (EPIRs) are mostly single-specialty systems with design configurations that allow their usage for cardiac ablation with catheter-tip deflection and distal-force sensing, catheter steerability, magnetic navigation, and advanced cardiac mapping, an essential consideration to control and navigate the steerable catheter to a desired position and orientation compared with vascular procedures [108,109,110,111][108][109][110][111]. This is applicable for the treatment of cardiac arrhythmia and atrial flutter. However, the review of literature in this restudyearch focuses on endovascular interventions, and profound description of electrophysiological interventions is reported in other literature [112]. Robot-assisted endovascular interventions are performed in two stages. The first stage is the manual procedure. The main task of the manual procedure is to provide a stable arterial access for the flexible surgical tool, followed by robotic navigation, which is usually performed by a doctor or assistant. The second stage is the robot-assisted procedures wherein the interventionalist remotely controls the robot to deliver guidewires and then the balloons and stent catheters with the aid of 2D real-time image data to the location of the vascular lesions. The two stages are illustrated in Figure 4 and Figure 5, respectively.Currently, commercial EVIRs specifically include the Corindus CorPath series, Hansen’s Magellan system, and Robocath’ s R-One robot system. Based on the robotic navigation system developed by Beyar et al. [23], Corindus developed the CorPath® 200, which was the first robotic system to receive an FDA approval for coronary interventions, in 2012. Subsequently, the CorPath® GRX system was approved by the FDA in 2016 to serve patients’ vascular needs with physicians’ opinions. The second-generation CorPath® GRX robotic system consists of an extended reach arm, a single-use disposable cassette, and a lead-shielded robotic-control workstation. As shown in Figure 6, this system can simultaneously manipulate the guidewire, guide–catheter balloon, and stent catheter. In addition, the precision and automation of the robot have been enhanced by the addition of “Dotter,” “Constant Speed,” “Active Device Fixation (ADF)” and “Auto Rotate-on-Retract” functions [113,114,115,116][113][114][115][116]. Overall, the robotic system is compact and could facilitate simplified manipulation of endovascular tools through the control work station; in addition, the drive cassette is a sterile disposable part that can be easily sterilized. However, the disadvantages are that it can only operate one set of guidewire and catheters, which is not fully capable for treating complex lesions, coupled with the high cost of consumables and the lack of distal force feedback [117,118][117][118]. The Hansen MagellanTM Robotic system is a remotely steerable catheter system that is based largely on the original Sensei® system but with significant modifications. It is composed of a remote device manipulator, physician workstation, and robotic catheter [76]. The remote device manipulator consists of two manipulators for operating flexible endovascular tools, and a drive friction belt that is designed for stabile tool delivery and distal tip control of the flexible endovascular tools, i.e., catheters and guidewires [16]. The Magellan system is designed for multi-specialty peripheral vascular procedures. To its merit, it is equipped with a self-developed controllable bending catheter. This facilitates smooth access to complex small peripheral vessels, provides rock-solid stability for guidewire delivery, and reduces the risk of vascular injury caused by contact and friction with the vessel wall [119]. However, the system’s disadvantage includes the need to manually place interventional devices (balloons, stents) after the surgical access has been established, the high cost of the robotic system, and the lack of haptic feedback [120].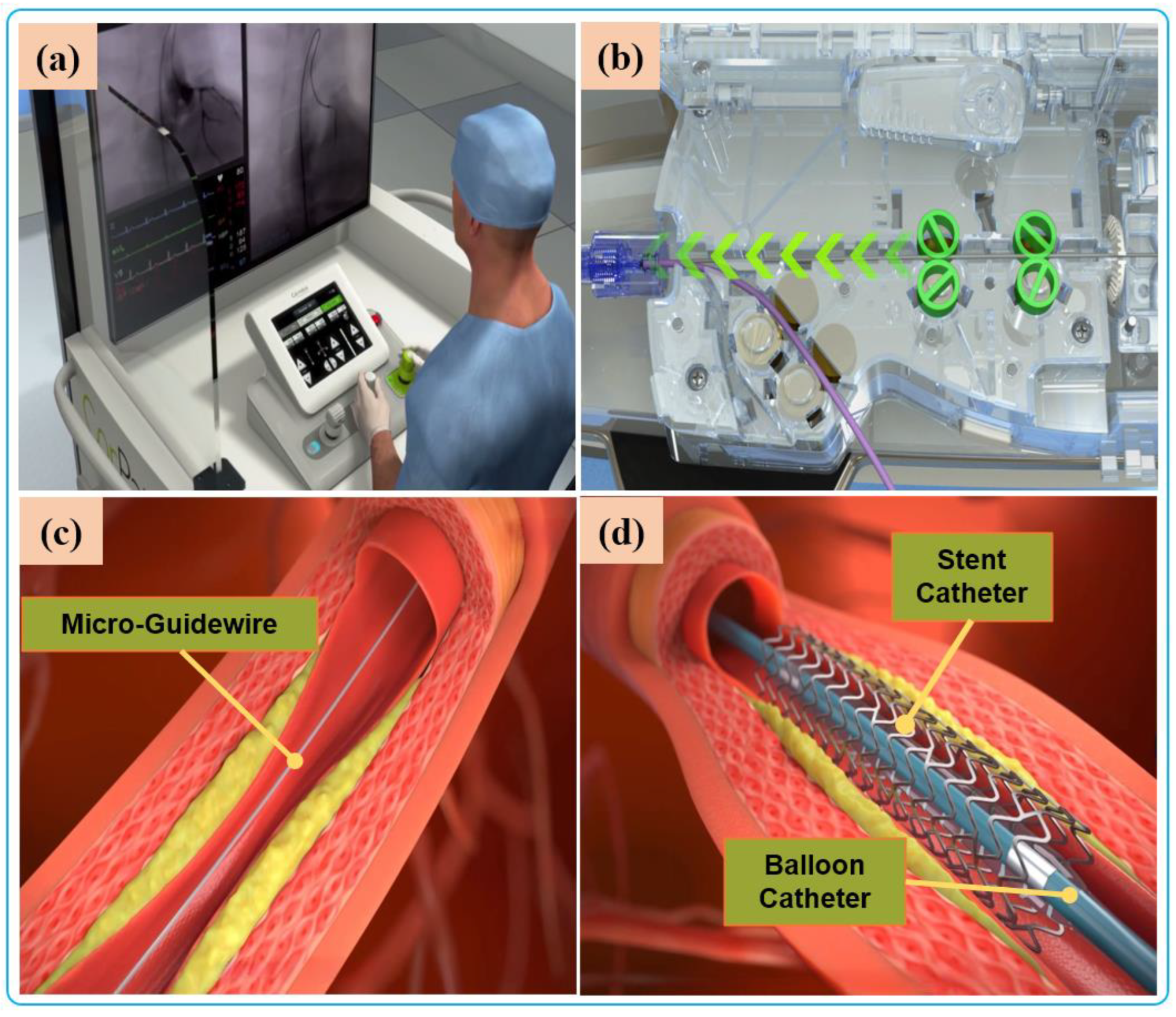 Figure 5. Robot-assisted procedures: (a) The interventionalist remote controls the robot on the console. (b) The robot delivers guidewires and catheters under the remote operation of the interventionalist. (c) Micro-guidewire passes the lesion site. (d) The stent is released to immobilize the lesion.Beyond the commercial levels, many research teams have conducted research on key technologies of robot-assisted vascular interventions. These are mostly found for force or haptic feedback, operation-safety strategies, and multi-device collaborative-delivery technology. Several VIRs have been developed within the research domain for these key technologies [121]. The ability of the robotic system to simultaneously manipulate multiple guidewires and catheters during the treatment of complex coronary lesions (type B2/C) is a key technology that is urgently needed in the cath lab and the focus of some existing studies. The treatment of complex lesions relies on the cooperative operation of multi-instruments. To achieve this, Ref. [122] designed two bionics to deliver both the catheter and guidewire simultaneously. The delivery of the guidewire is realized by the axial reciprocating motion, and the rotary motion is realized by three rollers that clamp the guidewire to each other and rotate in the same direction and speed. This device can realize the clamping and rotation of two guidewires. Only the crank rocker is interchanged to another set of rollers when switching between the guidewires. Although this mechanism can achieve multi-instrument movement, the volume and weight of the mechanism are usually large, and the clamping-wire part will form multi-segment bending, which may damage the instrument. In another study, Cha et al. [27] used a combination of linear reciprocating and rotating gear teeth to complete catheter translation and rotation, and used the combination of friction wheel and gear to drive the guidewire translation and rotation [82]. The authors carried out an in-vivo study to verify the robotic-system adaptability and functions for multi-instrument handling, control, and navigation [81]. The device has the advantages of easy disassembly and sterilization of component parts.
Figure 5. Robot-assisted procedures: (a) The interventionalist remote controls the robot on the console. (b) The robot delivers guidewires and catheters under the remote operation of the interventionalist. (c) Micro-guidewire passes the lesion site. (d) The stent is released to immobilize the lesion.Beyond the commercial levels, many research teams have conducted research on key technologies of robot-assisted vascular interventions. These are mostly found for force or haptic feedback, operation-safety strategies, and multi-device collaborative-delivery technology. Several VIRs have been developed within the research domain for these key technologies [121]. The ability of the robotic system to simultaneously manipulate multiple guidewires and catheters during the treatment of complex coronary lesions (type B2/C) is a key technology that is urgently needed in the cath lab and the focus of some existing studies. The treatment of complex lesions relies on the cooperative operation of multi-instruments. To achieve this, Ref. [122] designed two bionics to deliver both the catheter and guidewire simultaneously. The delivery of the guidewire is realized by the axial reciprocating motion, and the rotary motion is realized by three rollers that clamp the guidewire to each other and rotate in the same direction and speed. This device can realize the clamping and rotation of two guidewires. Only the crank rocker is interchanged to another set of rollers when switching between the guidewires. Although this mechanism can achieve multi-instrument movement, the volume and weight of the mechanism are usually large, and the clamping-wire part will form multi-segment bending, which may damage the instrument. In another study, Cha et al. [27] used a combination of linear reciprocating and rotating gear teeth to complete catheter translation and rotation, and used the combination of friction wheel and gear to drive the guidewire translation and rotation [82]. The authors carried out an in-vivo study to verify the robotic-system adaptability and functions for multi-instrument handling, control, and navigation [81]. The device has the advantages of easy disassembly and sterilization of component parts.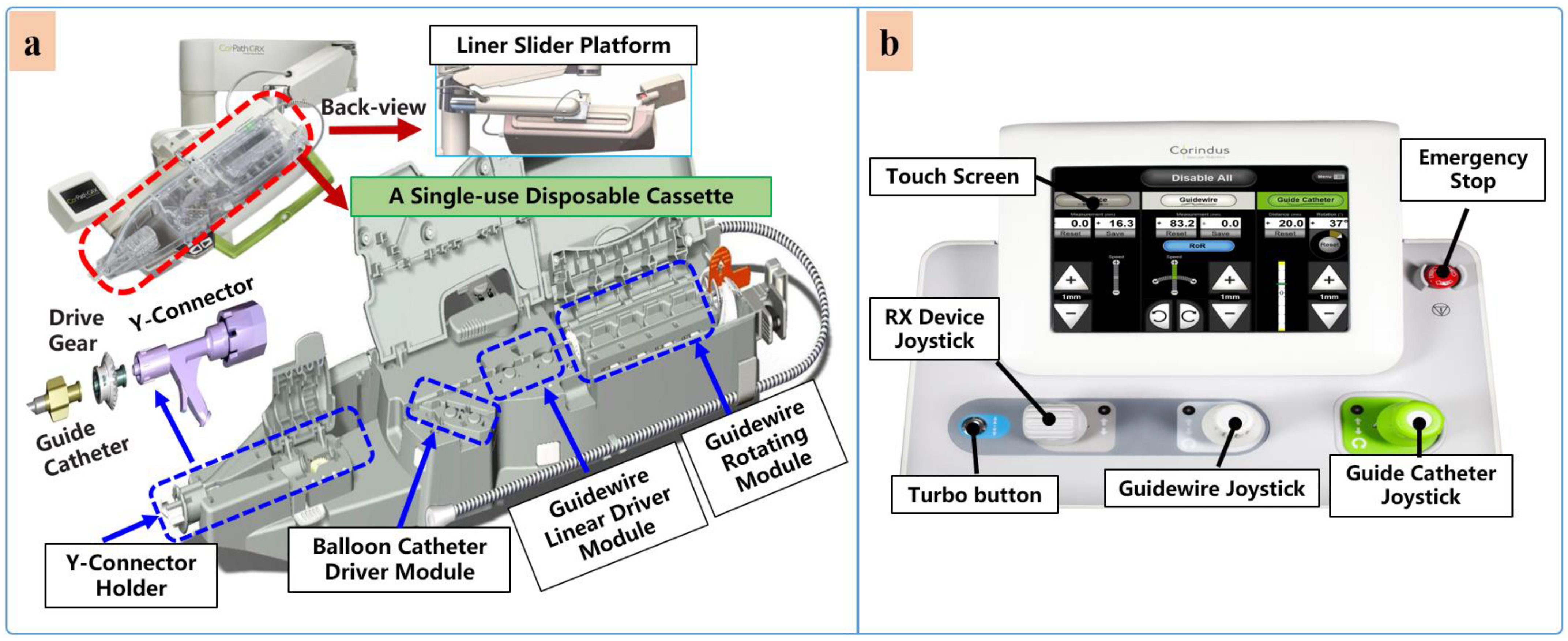 Figure 6. Commercial robotic system CorPath® GRX. (a) Bedside-instrument delivery mechanism includes an extended-reach arm and a single-use disposable cassette. (b) Robotic joystick controller for the CorPath® GRX system.
Figure 6. Commercial robotic system CorPath® GRX. (a) Bedside-instrument delivery mechanism includes an extended-reach arm and a single-use disposable cassette. (b) Robotic joystick controller for the CorPath® GRX system.
References
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of Plaque Formation and Rupture. Circ. Res. 2014, 114, 1852–1866.
- Rijanto, E.; Sugiharto, A.; Utomo, S.; Rahmayanti, R.; Afrisal, H.; Nanayakkara, T. Trends in robot assisted endovascular catheterization technology: A review. In Proceedings of the International Conference on Robotics, Biomimetics and Intelligent Computational Systems (Robionetics), Bali, Indonesia, 1 January 2017.
- Akinyemi, T.O.; Omisore, O.M.; Lu, G.; Wang, L. Toward a Fiber Bragg Grating-Based Two-Dimensional Force Sensor for Robot-Assisted Cardiac Interventions. IEEE Sens. Lett. 2022, 6, 1–4.
- Kumar, A.; Siddharth, V.; Singh, S.I.; Narang, R. Cost analysis of treating cardiovascular diseases in a super-specialty hospital. PLoS ONE 2022, 17, e262190.
- Akinyemi, T.O.; Omisore, O.M.; Duan, W.; Lu, G.; Al-Handerish, Y.; Han, S.; Wang, L. Fiber Bragg Grating-Based Force Sensing in Robot-Assisted Cardiac Interventions: A Review. IEEE Sens. J. 2021, 21, 10317–10331.
- Artico, M.; Spoletini, M.; Fumagalli, L.; Biagioni, F.; Ryskalin, L.; Fornai, F.; Salvati, M.; Frati, A.; Pastore, F.S.; Taurone, S. Egas Moniz: 90 Years (1927–2017) from Cerebral Angiography. Front. Neuroanat. 2017, 11, 81.
- Omisore, O.M.; Han, S.; Xiong, J.; Li, H.; Li, Z.; Wang, L. A Review on Flexible Robotic Systems for Minimally Invasive Surgery. IEEE Trans. Syst. Man Cybern. Syst. 2022, 52, 631–644.
- Zhao, Y.; Mei, Z.; Luo, X.; Mao, J.; Zhao, Q.; Liu, G.; Wu, D. Remote vascular interventional surgery robotics: A literature review. Quant. Imag. Med. Surg. 2021, 12, 2552–2574.
- Klein, L.W.; Tra, Y.; Garratt, K.N.; Powell, W.; Lopez-Cruz, G.; Chambers, C.; Goldstein, J.A. Occupational health hazards of interventional cardiologists in the current decade: Results of the 2014 SCAI membership survey. Catheter. Cardiovasc. Interv. 2015, 86, 913–924.
- Reeves, R.R.; Ang, L.; Bahadorani, J.; Naghi, J.; Dominguez, A.; Palakodeti, V.; Tsimikas, S.; Patel, M.P.; Mahmud, E. Invasive Cardiologists Are Exposed to Greater Left Sided Cranial Radiation the BRAIN Study (Brain Radiation Exposure and Attenuation during Invasive Cardiology Procedures). JACC Cardiovasc. Interv. 2015, 8, 1197–1206.
- Klein, L.W. Proper Shielding Technique in Protecting Operators and Staff from Radiation Exposure in the Fluoroscopy Environment. J. Invasive Cardiol. 2021, 33, E342–E343.
- Andreassi, M.G.; Piccaluga, E.; Guagliumi, G.; Del Greco, M.; Gaita, F.; Picano, E. Occupational Health Risks in Cardiac Catheterization Laboratory Workers. Circ. Cardiovasc. Interv. 2016, 9, e003273.
- Vano, E.; Kleiman, N.J.; Duran, A.; Romano-Miller, M.; Rehani, M.M. Radiation-associated Lens Opacities in Catheterization Personnel: Results of a Survey and Direct Assessments. J. Vasc. Interv. Radiol. 2013, 24, 197–204.
- Campbell, P.T.; Mahmud, E.; Marshall, J.J. Interoperator and intraoperator (in) accuracy of stent selection based on visual estimation. Catheter. Cardiovasc. Interv. 2015, 86, 1177–1183.
- Lo, N.; Gutierrez, J.A.; Swaminathan, R.V. Robotic-Assisted Percutaneous Coronary Intervention. Curr. Treat. Options Cardiovasc. Med. 2018, 20, 14.
- Antoniou, G.A.; Riga, C.V.; Mayer, E.K.; Cheshire, N.J.W.; Bicknell, C.D. Clinical applications of robotic technology in vascular and endovascular surgery. J. Vasc. Surg. 2011, 53, 493–499.
- Bonatti, J.; Vetrovec, G.; Riga, C.; Wazni, O.; Stadler, P. Robotic technology in cardiovascular medicine. Nat. Rev. Cardiol. 2014, 11, 266–275.
- Kazanzides, P.; Fichtinger, G.; Hager, G.D.; Okamura, A.M.; Whitcomb, L.L.; Taylor, R.H. Surgical and Interventional Robotics—Core Concepts, Technology, and Design . IEEE Robot. Autom. Mag. 2008, 15, 122–130.
- Akinyemi, T.O.; Omisore, O.M.; Chen, X.; Duan, W.; Du, W.; Yi, G.; Wang, L. Adapting Neural-Based Models for Position Error Compensation in Robotic Catheter Systems. Appl. Sci. 2022, 12, 10936.
- Rafii-Tari, H.; Payne, C.J.; Yang, G. Current and Emerging Robot-Assisted Endovascular Catheterization Technologies: A Review. Ann. Biomed. Eng. 2014, 42, 697–715.
- Granada, J.F.; Delgado, J.A.; Uribe, M.P.; Fernandez, A.; Blanco, G.; Leon, M.B.; Weisz, G. First-in-Human Evaluation of a Novel Robotic-Assisted Coronary Angioplasty System. JACC Cardiovasc. Interv. 2011, 4, 460–465.
- Weisz, G.; Metzger, D.C.; Caputo, R.P.; Delgado, J.A.; Marshall, J.J.; Vetrovec, G.W.; Reisman, M.; Waksman, R.; Granada, J.F.; Novack, V.; et al. Safety and Feasibility of Robotic Percutaneous Coronary Intervention. J. Am. Coll. Cardiol. 2013, 61, 1596–1600.
- Beyar, R.; Gruberg, L.; Deleanu, D.; Roguin, A.; Almagor, Y.; Cohen, S.; Kumar, G.; Wenderow, T. Remote-control percutaneous coronary interventions: Concept, validation, and first-in-humans pilot clinical trial. J. Am. Coll. Cardiol. 2006, 47, 296–300.
- Sankaran, N.K.; Chembrammel, P.; Siddiqui, A.; Snyder, K.; Kesavadas, T. Design and Development of Surgeon Augmented Endovascular Robotic System. IEEE Trans. Biomed. Eng. 2018, 65, 2483–2493.
- Thakur, Y.; Bax, J.S.; Holdsworth, D.W.; Drangova, M. Design and Performance Evaluation of a Remote Catheter Navigation System. IEEE Trans. Biomed. Eng. 2009, 56, 1901–1908.
- Guo, S.; Song, Y.; Yin, X.; Zhang, L.; Tamiya, T.; Hirata, H.; Ishihara, H. A Novel Robot-Assisted Endovascular Catheterization System with Haptic Force Feedback. IEEE Trans. Robot. 2019, 35, 685–696.
- Cha, H.J.; Yi, B.J.; Won, J.Y. An assembly-type master-slave catheter and guidewire driving system for vascular intervention. Proc. Inst. Mech. Eng. Part H 2017, 231, 69–79.
- Wang, H.; Chang, J.; Yu, H.; Liu, H.; Hou, C.; Lu, H. Research on a Novel Vascular Interventional Surgery Robot and Control Method Based on Precise Delivery. IEEE Access 2021, 9, 26568–26582.
- Mahmud, E.; Naghi, J.; Ang, L.; Harrison, J.; Behnamfar, O.; Pourdjabbar, A.; Reeves, R.; Patel, M. Demonstration of the Safety and Feasibility of Robotically Assisted Percutaneous Coronary Intervention in Complex Coronary Lesions Results of the CORA-PCI Study (Complex Robotically Assisted Percutaneous Coronary Intervention). JACC Cardiovasc. Interv. 2017, 10, 1320–1327.
- Mendes Pereira, V.; Cancelliere, N.M.; Nicholson, P.; Radovanovic, I.; Drake, K.E.; Sungur, J.; Krings, T.; Turk, A. First-in-human, robotic-assisted neuroendovascular intervention. J. Neurointerv. Surg. 2020, 12, 338–340.
- Weinberg, J.H.; Sweid, A.; Sajja, K.; Gooch, M.R.; Herial, N.; Tjoumakaris, S.; Rosenwasser, R.H.; Jabbour, P. Comparison of robotic-assisted carotid stenting and manual carotid stenting through the transradial approach. J. Neurosurg. 2020, 135, 21–28.
- Mahmud, E.; Schmid, F.; Kalmar, P.; Deutschmann, H.; Hafner, F.; Rief, P.; Brodmann, M. Feasibility and Safety of Robotic Peripheral Vascular Interventions: Results of the RAPID Trial. JACC Cardiovasc. Interv. 2016, 9, 2058–2064.
- van den Bruck, J.; Sultan, A.; Lüker, J.; Thomas, D.; Willems, S.; Weinmann, K.; Kuniss, M.; Hochadel, M.; Senges, J.; Andresen, D.; et al. Remote vs. conventional navigation for catheter ablation of atrial fibrillation: Insights from prospective registry data. Clin. Res. Cardiol. 2019, 108, 298–308.
- Cabrera-Rufino, M.; Ramos-Arreguín, J.; Rodríguez-Reséndiz, J.; Gorrostieta-Hurtado, E.; Aceves-Fernandez, M. Implementation of ANN-Based Auto-Adjustable for a Pneumatic Servo System Embedded on FPGA. Micromachines 2022, 13, 890.
- García-Martínez, J.R.; Cruz-Miguel, E.E.; Carrillo-Serrano, R.V.; Mendoza-Mondragón, F.; Toledano-Ayala, M.; Rodríguez-Reséndiz, J. A PID-Type Fuzzy Logic Controller-Based Approach for Motion Control Applications. Sensors 2020, 20, 5323.
- Odry, A.; Kecskes, I.; Csik, D.; Rodríguez-Reséndiz, J.; Carbone, G.; Sarcevic, P. Performance Evaluation of Mobile Robot Pose Estimation in MARG-Driven EKF, Cham. In Proceedings of the International Conference of IFToMM ITALY, Napoli, Italy, 7–9 September 2022; Springer: Cham, Switzerland, 2022.
- Shi, C.; Song, D.; Lai, D.; Wang, S. Development of a High-Sensitivity Proximal Force/Torque Sensor Based on Optical Sensing for Intravascular Robots. IEEE Trans. Med. Robot. Bionics 2022, 4, 957–966.
- Rafii-Tari, H.; Payne, C.J.; Bicknell, C.; Kwok, K.; Cheshire, N.J.W.; Riga, C.; Yang, G. Objective Assessment of Endovascular Navigation Skills with Force Sensing. Ann. Biomed. Eng. 2017, 45, 1315–1327.
- Zhou, X.H. Bian, G.B. Xie, X.L. Hou, Z.G. An Interventionalist-Behavior-Based Data Fusion Framework for Guidewire Tracking in Percutaneous Coronary Intervention. IEEE Trans. Syst. Man Cybern. 2020, 50, 4836–4849.
- Wang, T.; Zhang, D.; Liu, D. Remote-controlled vascular interventional surgery robot. Int. J. Med. Robot. Comp. 2010, 6, 194–201.
- Feng, Z.Q.; Bian, G.B.; Xie, X.L.; Hou, Z.G.; Hao, J.L. Design and evaluation of a bio-inspired robotic hand for percutaneous coronary intervention. In Proceedings of the IEEE International Conference on Robotics and Automation (ICRA), Seattle, WA, USA, 26–30 May 2015.
- Ghamraoui, A.K.; Ricotta, J.J. Current and Future Perspectives in Robotic Endovascular Surgery. Curr. Surg. Rep. 2018, 6, 21.
- Bian, G.; Xie, X.; Feng, Z.; Hou, Z.; Wei, P.; Cheng, L.; Tan, M. An enhanced dual-finger robotic Hand for Catheter manipulating in vascular intervention: A preliminary study. In Proceedings of the IEEE International Conference on Information and Automation (ICIA), Yinchuan, China, 26–28 August 2013.
- Wang, K.; Lu, Q.; Chen, B.; Shen, Y.; Li, H.; Liu, M.; Xu, Z. Endovascular intervention robot with multi-manipulators for surgical procedures: Dexterity, adaptability, and practicability. Robot. Comput.-Integr. Manuf. 2019, 56, 75–84.
- Shen, H.; Wang, C.; Xie, L.; Zhou, S.; Gu, L.; Xie, H. A novel remote-controlled robotic system for cerebrovascular intervention. Int. J. Med. Robot. Comp. 2018, 14, e1943.
- Choi, J.; Park, S.; Kim, Y.H.; Moon, Y.; Choi, J. A Vascular Intervention Assist Device Using Bi-Motional Roller Cartridge Structure and Clinical Evaluation. Biosensors 2021, 11, 329.
- Kassamali, R.H.; Ladak, B. The role of robotics in interventional radiology: Current status. Quant. Imag. Med. Surg. 2015, 5, 340–343.
- Payne, C.J.; Rafii-Tari, H.; Yang, G. A force feedback system for endovascular catheterization. In Proceedings of the IEEE/RSJ International Conference on Intelligent Robots and Systems, Algarve, Portugal, 7–12 October 2012.
- Rafii-Tari, H.; Payne, C.J.; Liu, J.; Riga, C.; Bicknell, C.; Yang, G.Z. Towards automated surgical skill evaluation of endovascular catheterization tasks based on force and motion signatures. In Proceedings of the IEEE International Conference on Robotics and Automation (ICRA), Seattle, WA, USA, 26–30 May 2015.
- Yu, H.; Wang, H.; Chang, J.; Niu, J.; Wang, F.; Yan, Y.; Tian, H.; Fang, J.; Lu, H. A Novel Vascular Intervention Surgical Robot Based on Force Feedback and Flexible Clamping. Appl. Sci. 2021, 11, 611.
- Omisore, O.M.; Akinyemi, T.O.; Du, W.; Duan, W.; Orji, R.; Do, T.N.; Wang, L. Weighting-Based Deep Ensemble Learning for Recognition of Interventionalists’ Hand Motions During Robot-Assisted Intravascular Catheterization. IEEE Trans. Hum.-Mach. Syst. 2022, 53, 1–13.
- Desai, V.R.; Lee, J.J.; Sample, T.; Kleiman, N.S.; Lumsden, A.; Britz, G.W. First in Man Pilot Feasibility Study in Extracranial Carotid Robotic-Assisted Endovascular Intervention. Neurosurgery 2021, 88, 506–514.
- Khan, E.M.; Frumkin, W.; Ng, G.A.; Neelagaru, S.; Abi-Samra, F.M.; Lee, J.; Giudici, M.; Gohn, D.; Winkle, R.A.; Sussman, J.; et al. First experience with a novel robotic remote catheter system: Amigo™ mapping trial. J. Interv. Card. Electr. 2013, 37, 121–129.
- Ma, Y.; Penney, G.P.; Bos, D.; Frissen, P.; de Fockert, G.; King, A.; Gao, G.; Yao, C.; Totman, J.; Ginks, M.; et al. Evaluation of a robotic arm for echocardiography to X-ray image registration during cardiac catheterization procedures. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009.
- Kim, J.; Lee, J.; Chung, J.W.; Shin, Y. Locally adaptive 2D–3D registration using vascular structure model for liver catheterization. Comput. Biol. Med. 2016, 70, 119–130.
- Matl, S.; Brosig, R.; Baust, M.; Navab, N.; Demirci, S. Vascular image registration techniques: A living review. Med. Image Anal. 2017, 35, 1–17.
- Tavallaei, M.A.; Lavdas, M.K.; Gelman, D.; Drangova, M. Magnetic resonance imaging compatible remote catheter navigation system with 3 degrees of freedom. Int. J. Comput. Ass. Rad. 2016, 11, 1537–1545.
- Guo, J.; Shi, C.; Ren, H. Ultrasound-Assisted Guidance with Force Cues for Intravascular Interventions. IEEE Trans. Autom. Sci. Eng. 2019, 16, 253–260.
- Bezerra, H.G.; Attizzani, G.F.; Sirbu, V.; Musumeci, G.; Lortkipanidze, N.; Fujino, Y.; Wang, W.; Nakamura, S.; Erglis, A.; Guagliumi, G.; et al. Optical Coherence Tomography Versus Intravascular Ultrasound to Evaluate Coronary Artery Disease and Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2013, 6, 228–236.
- Rosa, G.M.; Bauckneht, M.; Masoero, G.; Mach, F.; Quercioli, A.; Seitun, S.; Balbi, M.; Brunelli, C.; Parodi, A.; Nencioni, A.; et al. The vulnerable coronary plaque: Update on imaging technologies. Thromb. Haemost. 2013, 110, 706–722.
- Shi, C.; Luo, X.; Guo, J.; Najdovski, Z.; Fukuda, T.; Ren, H. Three-Dimensional Intravascular Reconstruction Techniques Based on Intravascular Ultrasound: A Technical Review. IEEE J. Biomed. Health 2018, 22, 806–817.
- Srimathveeravalli, G.; Kesavadas, T.; Li, X. Design and fabrication of a robotic mechanism for remote steering and positioning of interventional devices. Int. J. Med. Robot. Comp. 2010, 6, 160–170.
- Feng, Z.Q.; Hou, Z.G.; Bian, G.B.; Xie, X.L.; Zhou, X.H. Master-slave Interactive Control and Implementation for Minimally Invasive Vascular Interventional Robots. Acta Autom. Sin. 2016, 42, 696–705.
- Song, S.J.; Moon, Y.; Lee, D.H.; Ahn, C.B.; Jo, Y.; Choi, J. Comparative Study of Fuzzy PID Control Algorithms for Enhanced Position Control in Laparoscopic Surgery Robot. J. Med. Biol. Eng. 2015, 35, 34–44.
- Guo, J.; Jin, X.; Guo, S.; Du, W. Study on the tracking performance of the vascular interventional surgical robotic system based on the fuzzy-PID controller. In Proceedings of the IEEE International Conference on Mechatronics and Automation (ICMA), Takamatsu, Japan, 6–9 August 2017.
- Omisore, O.M.; Akinyemi, T.O.; Duan, W.; Du, W.; Wang, L. A Novel Sample-efficient Deep Reinforcement Learning with Episodic Policy Transfer for PID-Based Control in Cardiac Catheterization Robots. arXiv 2021, arXiv:211014941.
- Omisore, O.M.; Han, S.P.; Ren, L.X.; Wang, G.S.; Ou, F.L.; Li, H.; Wang, L. Towards Characterization and Adaptive Compensation of Backlash in a Novel Robotic Catheter System for Cardiovascular Interventions. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 824–838.
- Zhou, W.; Guo, S.; Guo, J.; Meng, F.; Chen, Z. ADRC-Based Control Method for the Vascular Intervention Master-Slave Surgical Robotic System. Micromachines 2021, 12, 1439.
- Ma, X.; Zhou, J.; Zhang, X.; Qi, Y.; Huang, X. Design of a New Catheter Operating System for the Surgical Robot. Appl. Bionics. Biomech. 2021, 2021, 8898311.
- Wu, D.; Zhang, Y.; Ourak, M.; Niu, K.; Dankelman, J.; Poorten, E.V. Hysteresis Modeling of Robotic Catheters Based on Long Short-Term Memory Network for Improved Environment Reconstruction. IEEE Robot. Autom. Lett. 2021, 6, 2106–2113.
- Kweon, J.; Kim, K.; Lee, C.; Kwon, H.; Park, J.; Song, K.; Kim, Y.I.; Park, J.; Back, I.; Roh, J.; et al. Deep Reinforcement Learning for Guidewire Navigation in Coronary Artery Phantom. IEEE Access 2021, 9, 166409–166422.
- Karstensen, L.; Ritter, J.; Hatzl, J.; Pätz, T.; Langejürgen, J.; Uhl, C.; Mathis-Ullrich, F. Learning-based autonomous vascular guidewire navigation without human demonstration in the venous system of a porcine liver. Int. J. Comput. Ass. Rad. 2022, 17, 2033–2040.
- Smitson, C.C.; Ang, L.; Pourdjabbar, A.; Reeves, R.; Patel, M.; Mahmud, E. Safety and Feasibility of a Novel, Second-Generation Robotic-Assisted System for Percutaneous Coronary Intervention: First-in-Human Report. J. Invasive Cardiol. 2018, 30, 152–156.
- Legeza, P.; Britz, G.W.; Loh, T.; Lumsden, A. Current utilization and future directions of robotic-assisted endovascular surgery. Expert Rev. Med. Devic. 2020, 17, 919–927.
- Madder, R.D.; VanOosterhout, S.; Parker, J.; Sconzert, K.; Li, Y.; Kottenstette, N.; Madsen, A.; Sungur, J.M.; Bergman, P. Robotic telestenting performance in transcontinental and regional pre-clinical models. Catheter. Cardiovasc. Interv. 2021, 97, E327–E332.
- Bismuth, J.; Kashef, E.; Cheshire, N.; Lumsden, A.B. Feasibility and Safety of Remote Endovascular Catheter Navigation in a Porcine Model. J. Endovasc. Ther. 2011, 18, 243–249.
- Bao, X.; Guo, S.; Xiao, N.; Li, Y.; Shi, L. Compensatory force measurement and multimodal force feedback for remote-controlled vascular interventional robot. Biomed. Microdevices 2018, 20, 74.
- Yang, X.; Wang, H.; Sun, L.; Yu, H. Operation and Force Analysis of the Guide Wire in a Minimally Invasive Vascular Interventional Surgery Robot System. Chin. J. Mech. Eng. 2015, 28, 249–257.
- Lu, W.S.; Xu, W.Y.; Pan, F.; Liu, D.; Tian, Z.M.; Zeng, Y. Clinical application of a vascular interventional robot in cerebral angiography. Int. J. Med. Robot. Comp. 2016, 12, 132–136.
- Wang, K.; Chen, B.; Xu, X. Design and control method of surgical robot for vascular intervention operation. In Proceedings of the IEEE International Conference on Robotics and Biomimetics (ROBIO), Qingdao, China, 3–7 December 2016.
- Song, H.; Woo, J.; Won, J.; Yi, B. In Vivo Usability Test of Vascular Intervention Robotic System Controlled by Two Types of Master Devices. Appl. Sci. 2021, 11, 5453.
- Woo, J.; Song, H.; Cha, H.; Yi, B. Advantage of Steerable Catheter and Haptic Feedback for a 5-DOF Vascular Intervention Robot System. Appl. Sci. 2019, 9, 4305.
- Abdelaziz, M.E.; Kundrat, D.; Pupillo, M.; Dagnino, G.; Kwok, T.M.; Chi, W.; Yang, G.Z. Toward a versatile robotic platform for fluoroscopy and mri-guided endovascular interventions: A pre-clinical study. In Proceedings of the IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Macau, China, 3–8 November 2019.
- Tanimoto, M.; Arai, F.; Fukuda, T.; Itoigawa, K.; Hashimoto, M.; Takahashi, I.; Negoro, M. Telesurgery system for intravascular neurosurgery. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Pittsburgh, PA, USA, 11–14 October 2000; Springer: Berlin/Heidelberg, Germany, 2000.
- Omisore, O.M.; Duan, W.; Du, W.; Zheng, Y.; Akinyemi, T.; Al-Handerish, Y.; Li, W.; Liu, Y.; Xiong, J.; Wang, L. Automatic tool segmentation and tracking during robotic intravascular catheterization for cardiac interventions. Quant. Imag. Med. Surg. 2021, 11, 2688–2710.
- Zhou, J.; Mei, Z.; Miao, J.; Mao, J.; Wang, L.; Wu, D.; Sun, D.; Zhao, Y. A Remote-Controlled Robotic System with Safety Protection Strategy Based on Force-Sensing and Bending Feedback for Transcatheter Arterial Chemoembolization. Micromachines 2020, 11, 805.
- Li, X.; Li, J.; Jing, J.; Ma, T.; Liang, S.; Zhang, J.; Mohar, D.; Raney, A.; Mahon, S.; Brenner, M.; et al. Integrated IVUS-OCT Imaging for Atherosclerotic Plaque Characterization. IEEE J. Sel. Top. Quantum Electron. 2014, 20, 196–203.
- Langsch, F.; Virga, S.; Esteban, J.; Gobl, R.; Navab, N. Robotic Ultrasound for Catheter Navigation in Endovascular Procedures. In Proceedings of the IEEE/RSJ International Conference On Intelligent Robots And Systems (IROS), Macau, China, 4–8 November 2019.
- Hasan, F.; Bonatti, J. Robotically assisted percutaneous coronary intervention: Benefits to the patient and the cardiologist. Expert Rev. Cardiovas. 2015, 13, 1165–1168.
- Akinyemi, T.O.; Omisore, O.M.; Duan, W.; Lu, G.; Du, W.; Alhanderish, Y.; Li, Y.; Wang, L. Development of a Millinewton FBG-Based Distal Force Sensor for Intravascular Interventions. In Proceedings of the International Conference on Control Automation Robotics and Vision (ICARCV 2020), Shenzhen, China, 13–15 December 2020.
- Reddy, V.Y.; Shah, D.; Kautzner, J.; Schmidt, B.; Saoudi, N.; Herrera, C.; Jaïs, P.; Hindricks, G.; Peichl, P.; Yulzari, A.; et al. The relationship between contact force and clinical outcome during radiofrequency catheter ablation of atrial fibrillation in the TOCCATA study. Heart Rhythm 2012, 9, 1789–1795.
- Guo, J.; Guo, S.; Yu, Y. Design and characteristics evaluation of a novel teleoperated robotic catheterization system with force feedback for vascular interventional surgery. Biomed. Microdevices 2016, 18, 76.
- Wei, W.; Yang, D.; Li, L.; Xia, Y. An Intravascular Catheter Bending Recognition Method for Interventional Surgical Robots. Machines 2022, 10, 42.
- Bandari, N.M.; Ahmadi, R.; Hooshiar, A.; Dargahi, J.; Packirisamy, M. Hybrid piezoresistive-optical tactile sensor for simultaneous measurement of tissue stiffness and detection of tissue discontinuity in robot-assisted minimally invasive surgery. J. Biomed. Opt. 2017, 22, 77002.
- Gan, L.; Duan, W.; Akinyemi, T.O.; Du, W.; Omisore, O.M.; Wang, L. Development of a Fiber Bragg Grating-based Force Sensor for Minimally Invasive Surgery—Case Study of Ex-vivo Tissue Palpation. IEEE Trans. Instrum. Meas. 2021, 1.
- He, C.; Wang, S.; Zuo, S. A linear stepping endovascular intervention robot with variable stiffness and force sensing. Int. J. Comput. Ass. Rad. 2018, 13, 671–682.
- Yan, Y.; Wang, H.; Yu, H.; Wang, F.; Fang, J.; Niu, J.; Guo, S. Machine Learning-based Surgical State Perception and Collaborative Control for a Vascular Interventional Robot. IEEE Sens. J. 2022, 22, 7106–7118.
- Bao, X.; Guo, S.; Guo, Y.; Yang, C.; Shi, L.; Li, Y.; Jiang, Y. Multilevel Operation Strategy of a Vascular Interventional Robot System for Surgical Safety in Teleoperation. IEEE Trans. Robot. 2022, 38, 2238–2250.
- Westebring-Van Der Putten, E.P.; Goossens, R.H.M.; Jakimowicz, J.J.; Dankelman, J. Haptics in minimally invasive surgery—A review. Minim. Invasive Ther. Allied Technol. 2008, 17, 3–16.
- Hooshiar, A.; Najarian, S.; Dargahi, J. Haptic Telerobotic Cardiovascular Intervention: A Review of Approaches, Methods, and Future Perspectives. IEEE Rev. Biomed. Eng. 2020, 13, 32–50.
- Hu, Z.; Zhang, J.; Xie, L.; Cui, G. A generalized predictive control for remote cardiovascular surgical systems. ISA Trans. 2020, 104, 336–344.
- Hasanzadeh, S.; Janabi-Sharifi, F. Model-based Force Estimation for Intracardiac Catheters. IEEE/ASME Trans. Mechatron. 2016, 21, 154–162.
- Amir, H.; Javad, A.; Naghmeh, B. Image-based Estimation of Contact Forces on Catheters for Robot-assisted Cardiovascular Intervention. In Proceedings of the Hamlyn Symposium on Medical Robotics, London, UK, 24–27 June 2018.
- Dagnino, G.; Liu, J.; Abdelaziz, M.E.; Chi, W.; Riga, C.; Yang, G.Z. Haptic feedback and dynamic active constraints for robot-assisted endovascular catheterization. In Proceedings of the IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Madrid, Spain, 1–5 October 2018.
- Guo, S.; Yamaji, H.; Kita, Y.; Izuishi, K.; Tamiya, T. A novel active catheter system for Ileus treatment. In Proceedings of the International Conference on Automation and Logistics, Qingdao, China, 1–3 September 2008.
- Shen, H.; Wang, C.; Xie, L.; Zhou, S.; Gu, L.; Xie, H. A novel robotic system for vascular intervention: Principles, performances, and applications. Int. J. Comput. Ass. Rad. 2019, 14, 671–683.
- Li, X.; Guo, S.; Shi, P.; Jin, X.; Kawanishi, M. An Endovascular Catheterization Robotic System Using Collaborative Operation with Magnetically Controlled Haptic Force Feedback. Micromachines 2022, 13, 505.
- Saliba, W.; Cummings, J.E.; Oh, S.; Zhang, Y.; Mazgalev, T.N.; Schweikert, R.A.; Burkhardt, J.D.; Natale, A. Novel robotic catheter remote control system: Feasibility and safety of transseptal puncture and endocardial catheter navigation. J. Cardiovasc. Electr. 2006, 17, 1102–1105.
- Kanagaratnam, P.; Koa-Wing, M.; Wallace, D.T.; Goldenberg, A.S.; Peters, N.S.; Davies, D.W. Experience of robotic catheter ablation in humans using a novel remotely steerable catheter sheath. J. Interv. Card. Electr. 2008, 21, 19–26.
- Krause, K.; Adamu, U.; Weber, M.; Hertting, K.; Hamm, C.; Kuck, K.; Hoffmann, R.; Kelm, M.; Blindt, R. German stereotaxis-guided percutaneous coronary intervention study group: First multicenter real world experience. Clin. Res. Cardiol. 2009, 98, 541–547.
- Bai, R.; Di Biase, L.; Valderrabano, M.; Lorgat, F.; Mlcochova, H.; Tilz, R.; Meyerfeldt, U.; Hranitzky, P.M.; Wazni, O.; Kanagaratnam, P.; et al. Worldwide Experience with the Robotic Navigation System in Catheter Ablation of Atrial Fibrillation: Methodology, Efficacy and Safety. J. Cardiovasc. Electr. 2012, 23, 820–826.
- Wellens, H. Catheter ablation for cardiac arrhythmias. N. Engl. J. Med. 2004, 351, 1172–1174.
- Beaman, C.B.; Kaneko, N.; Meyers, P.M.; Tateshima, S. A Review of Robotic Interventional Neuroradiology. Am. J. Neuroradiol. 2021, 42, 808–814.
- Walters, D.; Omran, J.; Patel, M.; Reeves, R.; Ang, L.; Mahmud, E. Robotic-Assisted Percutaneous Coronary Intervention. Interv. Cardiol. Clin. 2019, 8, 149–159.
- Sajja, K.C.; Sweid, A.; Al, S.F.; Chalouhi, N.; Avery, M.B.; Schmidt, R.F.; Tjoumakaris, S.I.; Gooch, M.R.; Herial, N.; Abbas, R.; et al. Endovascular robotic: Feasibility and proof of principle for diagnostic cerebral angiography and carotid artery stenting. J. Neurointerv. Surg. 2020, 12, 345–349.
- Panesar, S.S.; Britz, G.W. Endovascular Robotics: The Future of Cerebrovascular Surgery. World Neurosurg. 2019, 129, 327–329.
- Mahmud, E.; Pourdjabbar, A.; Ang, L.; Behnamfar, O.; Patel, M.P.; Reeves, R.R. Robotic technology in interventional cardiology: Current status and future perspectives. Catheter. Cardiovasc. Interv. 2017, 90, 956–962.
- Pourdjabbar, A.; Ang, L.; Reeves, R.R.; Patel, M.P.; Mahmud, E. The Development of Robotic Technology in Cardiac and Vascular Interventions. Rambam Maimonides Med. 2017, 8, e30.
- Rolls, A.; Riga, C. Endovascular robotics the current state and the future direction. Ann. R. Coll. Surg. 2018, 1007, 14.
- Riga, C.V.; Bicknell, C.D.; Rolls, A.; Cheshire, N.J.; Hamady, M.S. Robot-assisted Fenestrated Endovascular Aneurysm Repair (FEVAR) Using the Magellan System. J. Vasc. Interv. Radiol. 2013, 24, 191–196.
- Yu, H.Y.; Wang, H.B.; Zhang, W.; Liu, H.Y.; Chang, J.Y.; Huang, D. Master-slave system research of a vascular interventional surgical robot. In Proceedings of the IEEE International Conference on Real-time Computing and Robotics (RCAR), Kandima, Maldives, 1–5 August 2018.
- Wang, H.; Tian, H.; Guo, H.; Yang, X.; Tian, Y.; Hu, X.; Chen, J. Design of propulsion mechanism for vascular interventional operation robot. In Proceedings of the International Conference on Advanced Mechatronic Systems (ICAMechS), Zhengzhou, China, 30 August–2 September 2018.
More
