Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Ihtisham Ul Haq.
Phages are highly ubiquitous biological agents, which means they are ideal tools for molecular biology and recombinant DNA technology. The development of a phage display technology was a turning point in the design of phage-based vaccines. Phages are now recognized as universal adjuvant-free nanovaccine platforms. Phages are well-suited for vaccine design owing to their high stability in harsh conditions and simple and inexpensive large-scale production. Phage vaccines induce a strong and specific humoral response by targeted phage particles carrying the epitopes of SARS-CoV-2.
- phages
- COVID-19
- therapeutics
- variants
- phage display technology
- vaccines
1. CRISPR Engineering in Phage Vaccines against COVID-19
Very recently, viral components of SARS-CoV-2, such as envelope and capsid proteins, were inserted into a phage using CRISPR engineering, as shown in Figure 3. Segments of the SARS-CoV-2 genome were inserted in E. coli strains using the T4 genome phage, and each strain consisted of dual plasmids. Type 2 Cas9 and 5 Cas12a showed the genome-editing nucleases of the spacer plasmid. Furthermore, the protospacer sequences in the genome phage have a second plasmid (donor) which also consists of the SARS-CoV-2 sequence that is the target for the CRISPR RNA (crRNAs or the space RNA) [63][1]. The latter has homologous arms in the flanking position of approximately 500 base pairs long, used for the point of insertion. The genome has four non-essential regions that are used for the insertion of several SARS-CoV-2 genes. A universal vaccine platform that presents various important features was developed using phage T4 nanoparticles and CRISPR engineering with the exceptional advantage of generating rapid vaccines in any viral pandemic, including COVID-19 [28][2]. The use of type II Cas9 and type V Cas12a nucleases with CRISPR genome engineering created a sequence of recombinant SARS-CoV-2 gene insertions in a phage for the development of the T4 COVID-19 vaccine. In trials, antibody responses were broadly provoked by the T4 COVID-19 vaccine against various components, such as NP-specific and E-specific antigens, which confirmed the efficacy of the T4 COVID-19 vaccine [63][1]. During natural SARS-CoV-2 infection, T cells of the host immune system target NP-specific antigens [28][2]. Thus, specific and broader immune responses are triggered against multiple viral targets using a single-phage backbone in which various antigens are incorporated through the T4 CRISPR platform, as shown in Figure 41.
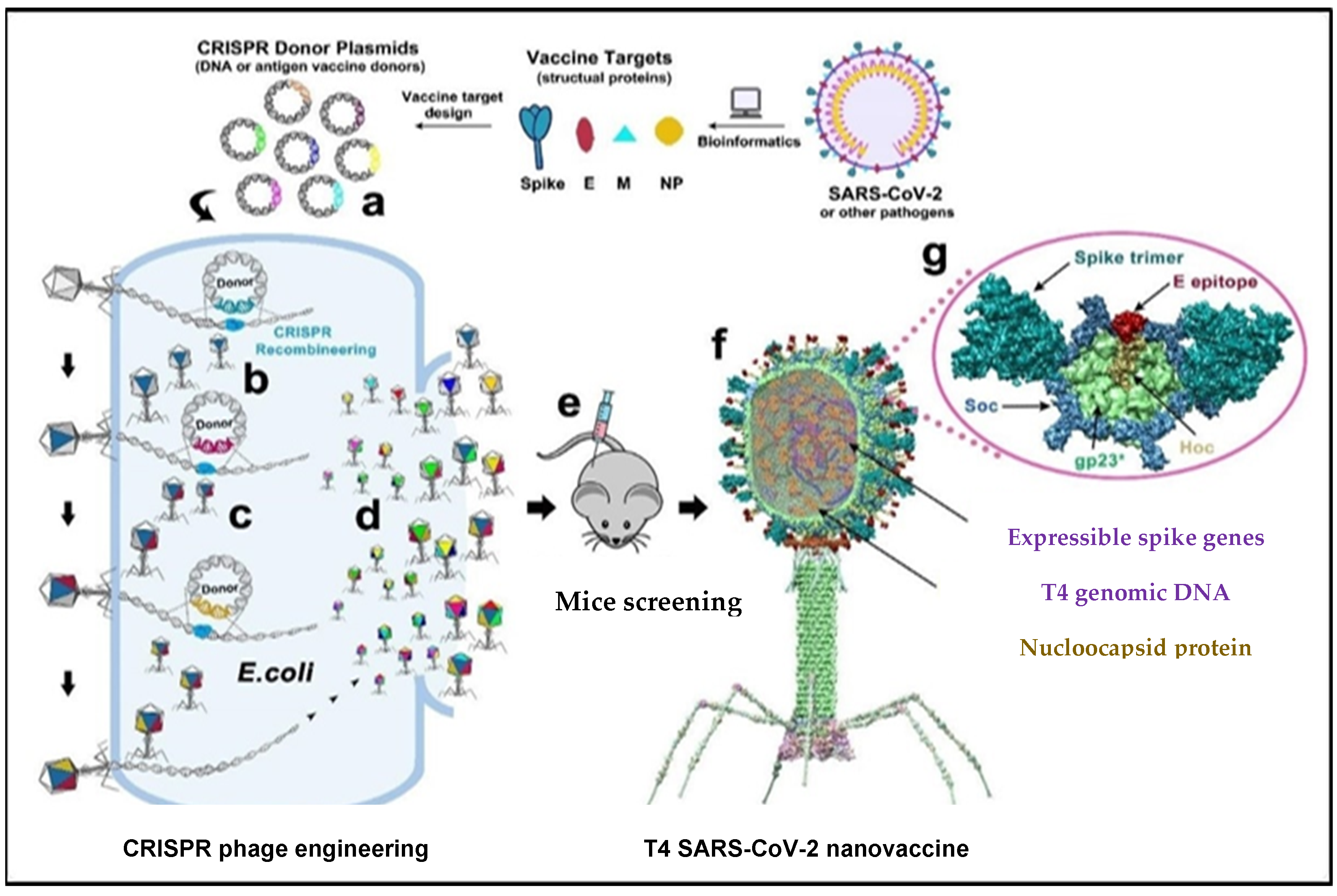
Figure 41. The genome of T4 phage was packed with engineered DNAs analogous to various components of SARS-CoV-2 virion. E. coli was grown and DNAs were inserted into it as a donor plasmid (a) CRISPR-targeted genome editing used for recpmbination into injected phage genome (b). Phage infections generated the several combinations of CoV-2 inserts and identified the recombinant phages in the progeny (c). For example, recombinant phage containing CoV-2 insert #1 (dark blue) can be used to infect CRISPR E. coli containing Co-V2 insert containing donor plasmid #2 (dark red). The progeny plaques obtained will contain recombinant phage #3 with both inserts #1 and #2 (dark blue plus dark red) in the same genome. This process was repeated to rapidly construct a pipeline of multiplex T4-SARS-CoV-2 vaccine phages (d). Selected vaccine candidates were then screened in a mouse model (e) to identify the most potent vaccine (f). Structural model of T4-SARS-CoV-2 Nanovaccine showing an enlarged view of a single 118 hexameric capsomer (g). The capsomer shows six subunits of major capsid protein gp23* (green), 119 trimers of Soc (blue), and a Hoc fiber (yellow) at the center of capsomer. The expressible spike genes are inserted into phage genome, the 12 aa E external peptide (red) is displayed at the tip of Hoc fiber, S-trimers (cyan) are attached to Soc subunits, and nucleocapsid proteins (yellow) are packaged in genome core. See Results and Materials and Methods for additional details. [28][2]. Reproduced with permission from [Venigalla B. Rao], [Science Advances]; published by [AAAS], [2021].
2. The Pharmacological Attributes of Phage Vaccines in COVID-19
The potential advantage of phage-based vaccines is the establishment of a staged approach with the capability of rapid modification in the vaccine in response to mutations. The induction of a broader immune response by a single-phage backbone renders it one of the best vaccine candidates to reduce the vaccine escape mutants significantly [62][3]. There is no need for adjuvants in phage vaccines, and the latitudinal nature of the exposed epitopes could be manipulated easily by the genetic and structural engineering of phages. The desired antigen could be combined with the phage backbone to create different formulations of the phage vaccine [63][1]. The diversity of the immune response could be increased in the same formulation carrying distinct and multiple antigens [64][4]. Phage display is a quick way to identify antibodies directed against any antigen of interest, and, as a result, this technology is already being used for the development of therapeutic antibodies. This necessitates the use of DNA from the beta cells isolated from people who have already developed the necessary antibodies. The European Union and the United States of America are both running initiatives to collect convalescent plasma with antibodies that target SARS-CoV-2 [65][5].
It is estimated that a few hundred thousand to 1,000,000 phage vaccine doses against SARS-CoV-2 could be formed in a 100-L fermenter, thus leading to large-scale production. Phage vaccines would be a solid possibility for extensively defensive COVID-19 vaccines and booster immunizations to previously vaccinated people [28,66][2][6]. Conventional vaccines developed against COVID-19 are generally effective in the prevention of infectious diseases, but their lack of stability raises concerns with transport, safety, and targeted delivery [67][7]. Phages are exceptionally stable nanoparticles, have a great profile of safety, and may be produced at moderately minimal costs. Phages are non-pathogenic to humans, have negligible reports of infections, and stimulate the eukaryotic immune system with potential adjuvant abilities [63][1]. They provide a new and strong elective stage to quickly produce viable antibodies against any pestilence or pandemic later on, especially when multivalent immunizations are crucial for controlling future pandemics and securing worldwide networks. The adaptability and flexibility of the phage vaccine are the potential benefits that could conquer the emerging variants of SARS-CoV-2. Phage vaccine efficacy could be increased even more by combining the spike and nucleocapsid proteins, and it would be more effective against the current and future variants of SARS-CoV-2 [68][8]. In the context of vaccine design, phage display is a fast, low-cost, reliable, and high-throughput method for the screening and selection of peptide antigens in an effective and straightforward manner [69][9]. These approaches are fast, inexpensive, highly adaptable, and easy to use.
3. Safety and Biocompatibility Concerns with Phage Vaccines
Favorable safety measures in phage vaccines are the key feature principally contributing to the therapeutic activities of phages [51][10], and the immunized organism is at lower risk of phage-conferred pathogenicity. The flexibility in the route of administration of the phage vaccine is another possible advantage, which could be either mucosal, intramuscular, or oral based on the adaptability to virus mutations [69][9]. Additionally, phages protect host cells by competing with the SARS-CoV-2 virus for surface assimilation and penetration, thus acting as a shield for eukaryotic cells. The pandemic burden could be reduced by phage-based vaccines whose safety profiles are well-recognized and not expensive compared to other vaccines [70][11]. Therefore, the burden of the emergence and circulation of SARS-CoV-2 variants could be reduced through the development of phage-based vaccines. Despite the successful presentation of peptides in phages, the major limitation of the development of these vaccines involves the accurate display of these molecules on the surface of the phage [51][10]. This problem revolves around two major aspects: the correct folding of a polypeptide chain and the sufficient display of functional epitopes. Choosing a peptide with the right strong antigenic characteristics is essential. However, a major difficulty is finding the right size of the peptide to be fused with the phage peptide without affecting the structure and other properties of the phage [71][12]. The efficiency of phage vaccines is dependent on both these properties in order to produce a significant immune response [47][13].
Despite being labeled as safe, there is an active possibility of bacterial infection in the gut if orally administrated. Phages may infect the bacteria in the gut, which could release harmful toxins to the host [72][14]. The size of the antigen should be small as large peptides cannot be accommodated into the tail tube or capsid; thus, a large antigen could be a hindrance in phage vaccine development. Moreover, the size of the genome should also be considered, as the length of the genome and the packaging capacity of the virion should overlap [73][15]. However, this limitation is evaded with certain other platforms [28][2]. Some studies have reported the alteration in the structure of the peptide upon insertion of the SARS-CoV spike epitopes into the phage. This poses another challenge in the production of phage-based vaccines. It was speculated that the epitopes with the minimum number of alterations in the normal structure are most efficient in producing the immune response [61][16]. In conclusion, the behavior of the immune system in response to such vaccines and the exhibition of peptides in the suitable confirmation for the maximum immunological response remain open questions for the successful designing of such vaccines.
Virus-like particles (VLPs)were also reported as an effective and safe platform for vaccine design and development. However, it is difficult to produce clinically viable VLPs [74][17]. Compared with subunit vaccines, VLPs have higher stability. Though, they are not stable during downstream processing and when environmental conditions change, as they lack viral genetic material [75][18]. There are stability concerns reported for VLP vaccines already available in the marketplace. The integrity of VLPs is altered due to the impact of temperature changes, agitation rates, fluid dynamics, and chemical treatments [76][19]. The immunogenicity of VLPs significantly reduces due to structural breakdowns. It also interferes with cell growth and the production of metabolic proteins, which impacts VLP production.
References
- Elahi, Y.; Mazaheri Nezhad Fard, R. Phage Therapy in COVID-19 Treatment. Int. J. Infect. 2022, 9, e116630.
- Zhu, J.; Ananthaswamy, N.; Jain, S.; Batra, H.; Tang, W.C.; Lewry, D.A.; Rao, V.B. A universal bacteriophage T4 nanoparticle platform to design multiplex SARS-CoV-2 vaccine candidates by CRISPR engineering. Sci. Adv. 2021, 7, eabh1547.
- Davenport, B.J.; Catala, A.; Weston, S.M.; Johnson, R.M.; Ardanuy, J.; Hammond, H.L.; Dillen, C.; Frieman, M.B.; Catalano, C.E.; Morrison, T.E. Phage-like particle vaccines are highly immunogenic and protect against pathogenic coronavirus infection and disease. npj Vaccines 2022, 7, 1–15.
- Chen, Y.; Batra, H.; Dong, J.; Chen, C.; Rao, V.B.; Tao, P. Genetic engineering of bacteriophages against infectious diseases. Front. Microbiol. 2019, 10, 954.
- Martinecz, A.; Wojewodzic, M.W. Could bacteriophages be the answer to the COVID-19 crisis? Exp Rev. Anti-Infect. Ther 2021, 19, 557–558.
- Ng, W.H.; Liu, X.; Mahalingam, S. Development of vaccines for SARS-CoV-2. F1000Research 2022, 9, 991.
- Excler, J.L.; Saville, M.; Berkley, S.; Kim, J.H. Vaccine development for emerging infectious diseases. Nat. Med. 2021, 27, 591–600.
- Zalewska-Piątek, B.; Piątek, R. Bacteriophages as Potential Tools for Use in Antimicrobial Therapy and Vaccine Development. Pharmaceuticals 2021, 14, 331.
- Huang, J.; Ding, Y.; Yao, J.; Zhang, M.; Zhang, Y.; Xie, Z.; Zuo, J. Nasal Nanovaccines for SARS-CoV-2 to Address COVID-19. Vaccines 2022, 10, 405.
- Slupetzky, K.; Gambhira, R.; Culp, T.D.; Shafti-Keramat, S.; Schellenbacher, C.; Christensen, N.D.; Roden, R.B.; Kirnbauer, R.A. A papillomavirus-like particle (VLP) vaccine displaying HPV16 L2 epitopes induces cross-neutralizing antibodies to HPV11. Vaccine 2007, 25, 2001–2010.
- Singh, A.K.; Gaur, V.; Kumar, A. Role of Phage Therapy in COVID-19 Infection: Future Prospects. In Bacteriophages in Therapeutics; IntechOpen: London, UK, 2021.
- Piekarowicz, A.; Kłyż Stein, D.C. A New Vaccination Method Based on Phage NgoΦ6 and Its Phagemid Derivatives. Front. Microbiol. 2022, 13, 793205.
- Samoylova, T.I.; Norris, M.D.; Samoylov, A.M.; Cochran, A.M.; Wolfe, K.G.; Petrenko, V.A.; Cox, N.R. Infective and inactivated filamentous phage as carriers for immunogenic peptides. J. Virol. Methods 2012, 183, 63–68.
- Loc-Carrillo, C.; Abedon, S.T. Pros and cons of phage therapy. Bacteriophage 2011, 1, 111–114.
- Freeman, D.; Waite, F.; Rosebrock, L.; Petit, A.; Causier, C.; East, A.; Jenner, L.; Teale, A.L.; Carr, L.; Mulhall, S.; et al. Coronavirus conspiracy beliefs, mistrust, and compliance with government guidelines in England. Psychol. Med. 2022, 52, 251–263.
- Staquicini, D.I.; Tang, F.H.; Markosian, C.; Yao, V.J.; Staquicini, F.I. Design and proof-of-concept 363 for targeted phage-based COVID-19 vaccination strategies with a streamlined cold-free 364 supply chain. Proc. Natl. Acad. Sci. 2021, 118, e2105739118.
- Charlton Hume, H.K.; Vidigal, J.; Carrondo, M.J.; Middelberg, A.P.; Roldão, A.; Lua, L.H. Synthetic biology for bioengineering virus-like particle vaccines. Biotechnol. Bioeng. 2019, 116, 919–935.
- Dai, S.; Wang, H.; Deng, F. Advances and challenges in enveloped virus-like particle (VLP)-based vaccines. J. Immunol. Sci. 2018, 2, 36–41.
- Roldao, A.; Silva, A.C.; Mellado, M.C.M.; Alves, P.M.; Carrondo, M.J.T. Viruses and virus-like particles in biotechnology: Fundamentals and applications. Compr. Biotechnol. 2011, 1, 633–656.
More
