The biological nature of the various populations of circulating tumor cells (CTCs) within the blood is still not well understood. Tumor cell fusion with immune cells is a longstanding hypothesis that has caught more attention in recent times. Specifically, fusion of tumor cells with macrophages might lead to the development of metastasis by acquiring features such as genetic and epigenetic heterogeneity, chemotherapeutic resistance, and immune tolerance. A unique circulating cell population has been identified as being potential fusions cells, characterized by distinct, large, polymorphonuclear cancer-associated cells with a dual epithelial and macrophage phenotype. Artificial fusion of tumor cells with macrophages leads to migratory, invasive, and metastatic phenotypes. Further studies might investigate whether these have a potential impact on the immune response towards the cancer. Such fusion cells could be a key component in cancer metastasis, and therefore, evolve as a diagnostic and therapeutic target in cancer precision medicine.
- Cancer
- tumor fusion cells
- macrophages
- liquid biomarkers
- circulating tumor cells
1. Introduction
Recent reports on circulating cancer-associated cells with both epithelial and macrophage/myeloid phenotypes in cancer patients, combined with genetic evidence, have supported the idea that fusion has a critical role in cancer progression (Figure 1)[1][2][3][4] [12,15,16,56].
2. Fusion of Tumor Cells with Macrophages
Macrophage M1 or M2 polarization appears to be critical for various aspects of immune responses to cancer and its progression[5] [57]. Macrophage infiltration of the primary tumor and polarization depend on cytokines in the tumor microenvironment (TME)[6] [58]. Within the TME, tumor-associated macrophage polarization to the M1 phenotype can be triggered through bacterial lipopolysaccharide (LPS) and by T helper 1 (Th1) cytokines, such as IFN-γ and also by TNF-α[7] [59]. The M1 phenotype is associated with anti-tumor properties[8] [60]. M2 phenotype macrophages have pro-tumoral effects, leading to increased cancer cell survival, proliferation, invasiveness, and immunosuppression in favor of the tumor[5] [57]. M2 polarization is induced by T helper 2 cytokines interleukin (IL)-4, IL-13, macrophage colony-stimulating factor (M-CSF), and transforming growth factor (TGF)-β[8] [60]. M2 macrophages are anti-inflammatory, immunosuppressive, and promote cancer progression, chemoresistance, and metastasis[9][10] [61,62]. M2 macrophages have critical interactions with tumor cells, but also with cells associated with tumor progression, such as Th2 cells, cancer-associated fibroblasts, regulatory T cells (Tregs), and myeloid-derived suppressor cells[5] [57]. M2 polarization phenotypes have also been observed in tumor fusion cells[11] [63]. Importantly, macrophages also have a high fusogenic potential, which is also likely to occur with tumor cells[12][13][8][14] [18,20,58,64]. In vitro and in vivo studies suggest that metastatic cells can be the result of the fusion of tumor cells with cells of hematopoietic/myeloid lineage, specifically with macrophages[15][12][16][13][17][18][19][20] [17–22,34,54]. Importantly, patient-derived tumor-macrophage fusion cells were shown to have M2 macrophage phenotypes[17][18] [21,22]. In a murine melanoma metastasis model, certain clones of lung metastasis cells had properties of melanoma cell—macrophage fusion cells[21] [65]. Importantly, fusion of tumor cells with macrophages is supported through the observation of these fusion cells in cancer patients[17][18][22][23] [21,22,27,28]. Macrophage fusion receptor DAP12 expression is associated with higher metastatic rates in breast cancer patients[13][24][25] [20,66,67]. It remains unclear whether tumor-associated macrophages fuse within the tumor microenvironment at the site of the tumor, in the blood, or in the lymphatic system. Understanding molecular fusion mechanisms between macrophages and tumor cells and the impact that fusion cells have on the immune system is of high interest in identifying therapeutic targets.
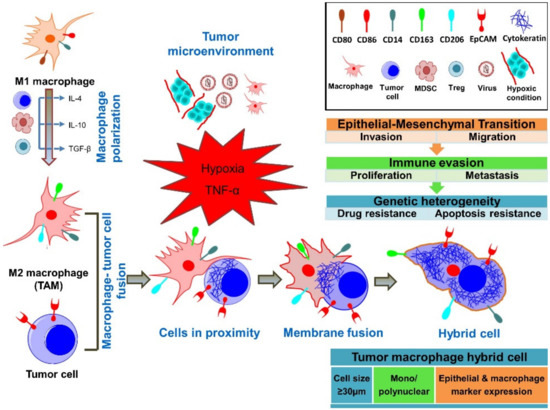
Figure 1. Concepts of fusion between tumor cells and macrophages. It is hypothesized that tumor-associated M2-polarized macrophages (TAMs) fuse their membranes with tumor cells, forming a tumor–macrophage hybrid cell. These fusion cells are large, mononuclear/polynuclear, and express both epithelial and myeloid markers. Importantly, fusion cells exert pro-tumorigenic and pro-metastatic effects through the outlined mechanisms.
