You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Fedor Zubkov.
Each metabolite, regardless of its molecular simplicity or complexity, has a mission or function in the organism biosynthesizing it. The biological, allelochemical, and chemical properties of acetophenone, as a metabolite involved in multiple interactions with various (mi-cro)organisms.
- acetophenones
- secondary metabolites
- toxicity
- Biosynthesis
- repellent
- attractant
- ketone
- natural molecules.
1. Introduction
In a broad context, nature provides us with various “typical” life components, such as air, water, and food, enhancing our well-being and generously giving us the essentials for our survival. Reducing the focus to a scientific level, nature teaches us, sparks creativity, and improves problem-solving skills. One of the most valuable gifts from nature, i.e., from all living (micro)organisms, is diverse natural products with complex molecular architectures, sophisticated mechanisms of action, and extraordinary biopotentiality that can be converted into innovative leads or drugs [1,2][1][2]. However, each complex or simple molecule generated in nature acts as a metabolite for survival and reproduction, functioning as a small screw in the machinery of the biological systems of living organisms.
While primary metabolites have essential metabolic roles in nutrition and reproduction, secondary metabolites are nonessential to life but contribute to the suitability of the species for survival. Low-molecular-weight compounds, called small molecules, are often involved in defense mechanisms [3[3][4],4], i.e., the protection of vegetable or animal organisms against biotic or abiotic stresses, and are used as special chemicals, such as drugs, flavors, fragrances, insecticides, and dyes, by humans owing to their great economic value. OIn this minireview, one specific small molecule will be discussed—acetophenone (1) (Figure 1). Acetophenone has earned this honor due to its importance in chemical, ecological, biological, and medicinal areas of science owing to its extraordinary and irreplicable chemical, biological, pharmacological, and allelochemical properties.
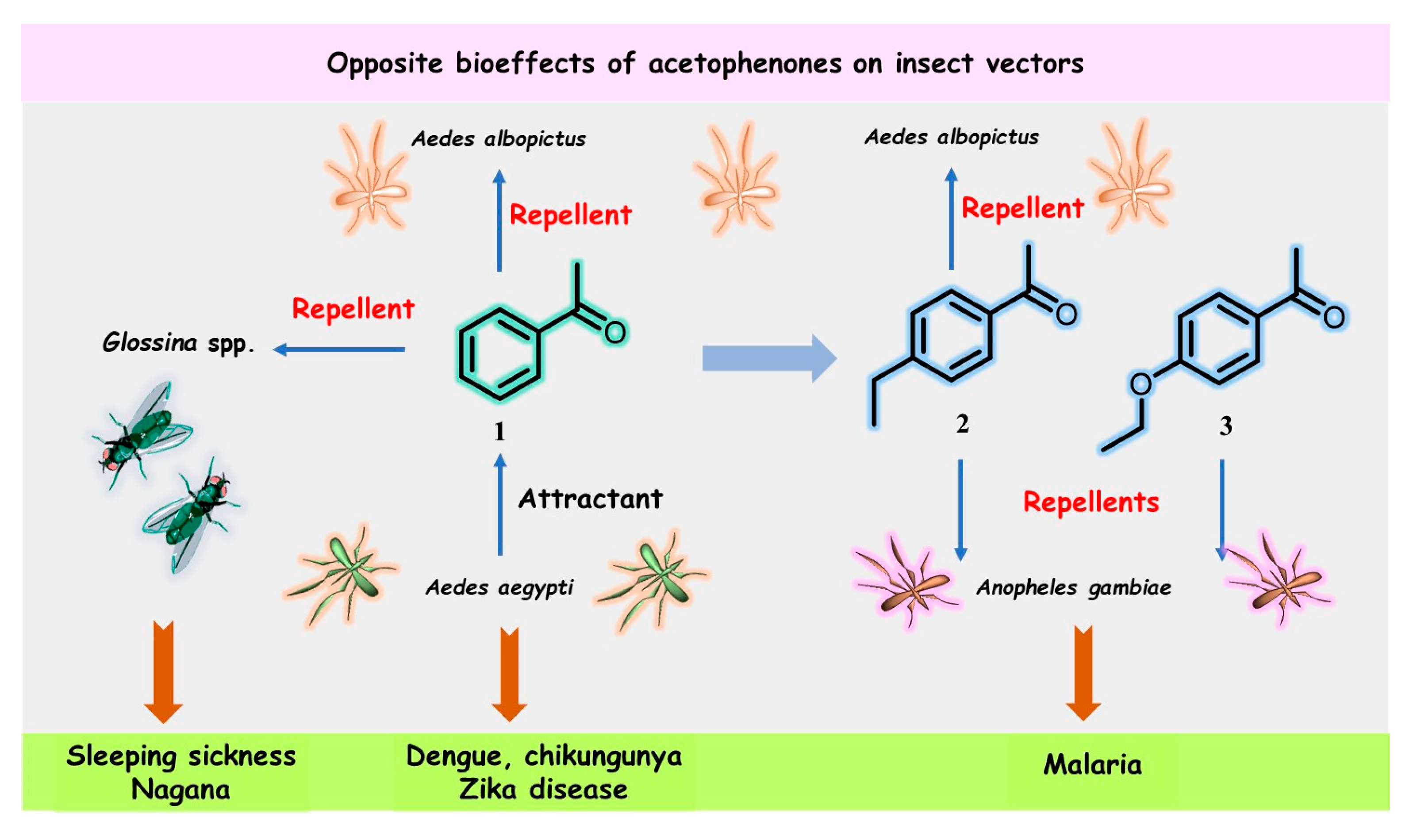
Figure 1. Structures and opposite bioeffects of acetophenone (1) and its derivatives 2 and 3 on insect vectors of dangerous tropical infectious diseases, including malaria, sleeping sickness, chikungunya, dengue, and Zika virus, transmitted by mosquitoes, flies, or ticks.
2. Acetophenone Is One of the Most Interesting Players in Skin Microbiota Manipulation by Vector-Borne Parasites Found in Animal-Feeding Insects
In July 2022, Cheng et al. reported that acetophenone released from the skin microbiota of flavivirus-infected hosts (mice and patients with dengue fever) acts as a potent attractant for Aedes mosquitoes, which are vectors for dengue (DENV2), chikungunya (CHIKV), and Zika (ZIKV) virus transmission, thereby increasing flavivirus transmission to aggravate dengue, chikungunya, and Zika diseases [11][5]. Aedes mosquitoes prefer to feed on mice infected by dengue and Zika viruses due to the released acetophenone compared to uninfected mice in control groups, in which acetophenone is absent. Female Aedes aegypti (Ae. aegypti) and Aedes albopictus (Ae. albopictus) mosquitoes were tested in these experiments, which showed that flaviviruses can stimulate the proliferation of acetophenone-producing skin commensal bacteria (especially the Bacillus genus) by suppressing the expression of the essential antimicrobial resistin-like molecule-α (RELMα) protein that protects against pathogenic bacterial skin infections, thereby killing the microbes. In particular, viruses can change host odors to attract mosquitoes.
It is worth noting that the use of isotretinoin, a naturally occurring retinoic acid and a vitamin A derivative, reduces attractiveness to mosquitoes through the activation of the expression of the RELMα protein in the skin of flavivirus-infected animals [11][5]. This interesting and important finding caught the attention of scientists who made brief but substantial and picturesque comments on Cheng’s work in prestigious journals such as Science [12][6], Nature Reviews Microbiology [13][7], and Cell [14][8]. Regardless, wresearchers wanted to examine this simple ketone from the perspective of synthetic and applied organic chemistry and chemical ecology fields.
It should be noted that the connection between the skin microbial population and attractiveness to mosquitoes and other blood-sucking insects has been studied, for example, in the African malaria mosquito Anopheles gambiae (An. gambiae) (infected with Plasmodium falciparum sporozoites), which plays an important role in malaria transmission [15,16[9][10][11][12],17,18], and the triatomine bug Rhodnius prolixus, which is the main vector transmitting the parasite Trypanosoma cruzi, the causative agent of Chagas disease [19,20][13][14]. Their host-seeking behavior is influenced by host scents, i.e., appropriate concentrations of volatile odors (a mixture of molecules, so-called molecular stimuli, such as ammonia, isobutylamine, acetone, lactic acid, isobutyric acid, and CO2) [19][13] present in human (or animal) skin microflora that can activate, attract, and even repel these blood-sucking insects.
Blood-sucking insects have excellent sensory abilities for detecting and following the physical and chemical signals (semiochemicals) emitted by their hosts; Aedes mosquitoes are not an exception. Cheng’s work [11][5] showed that the volatile acetophenone in the host skin microbiota produced a considerable electrophysiological response detectable by the antenna of Ae. aegypti, and thus, high levels of acetophenone attracted more mosquitoes than uninfected mice and healthy individuals in control groups. Nevertheless, close derivatives of acetophenone (1), 4-ethylacetophenone (2), and 4-ethoxyacetophenone (3) (Figure 1) had repellent effects on the malaria mosquito An. gambiae [18,21][12][15].
Moreover, acetophenone (1) and its para-ethyl analog 2 were found to exhibit significant repellency for the male Asian tiger mosquito, Ae. albopictus, but only at a high concentration (10−2) [22][16]. The latter metabolite has been described as a male-swarming aggregation pheromone for Ae. aegypti mosquitoes [23][17], which increases female attraction and mating success. However, none of the three acetophenone derivatives 1–3 were identified among specific semiochemicals, i.e., acetoin (3-hydroxy-2-butanone), sulcatone (6-methyl-5-hepten-2-one), octanal, nonanal, and decanal, released from the males of Anopheline species—Anopheles arabiensis and An. gambiae [24][18]. Such pheromones can remarkably manipulate these highly dangerous insects.
On the other hand, acetophenone (1) was found to be an effective repellent in field experiments [25][19] for tsetse flies (Glossina spp.) infected by Glossina hytrosavirus, the primary vector of African trypanosomes, which cause human African trypanosomosis (HAT or sleeping sickness) and African animal trypanosomosis (AAT or nagana) [26][20]. Recently, it was reported that acetophenone-containing Zebra skin odor repels the savannah tsetse fly [27[21][22],28], confirming previous results from field experiments [25][19]. Therefore, the diametrically opposite bioeffects of acetophenone (1) mentioned above could be used in the control and prevention of heralded diseases. This volatile ketone could be a suitable biomarker for detecting pathogens that actively manipulate host odors. However, the development of these volatile-based diagnostics, which use the “signal” of disease and the background “noise” of genetic and environmental variations, is still in its infancy [29,30][23][24]. On the other hand, odor-bait technology as a surveillance and control tool for insect vectors, such as tsetse flies or Aedes mosquitoes, has been promoted as a new and viable component of the integrated vector management program [31,32,33][25][26][27]. In this context, some extracts and essential oils from aromatic plants could be more advantageous in the biological control of pest insects. Nonetheless, there is some skepticism over this aspect [34][28].
Another analog of acetophenone, i.e., 2-aminoacetophenone, secreted by Pseudomonas aeruginosa, which is a ubiquitous opportunistic human pathogen, can facilitate attraction to food for several fly species, including Musca domestica, Ceratitis capitata, and Drosophila melanogaster [35][29].
3. Acetophenone Is a Prolific Semiochemical for Plant-Feeding Insects in Complex Interspecific Communication
Investigations on the involvement of plant volatiles in the feeding attraction behavior of mosquitoes were conducted before Chang’s work [11][5]. Mosquitoes visit flowers for nectar and may, in turn, act as pollinators for plants [36][30]. Like animal skin microflora, the composition of the inflorescence odor of plants can be an attractant or a repellent for mosquitoes [37][31].
Gonzalez-Audino et al. demonstrated that freshly cut inflorescences of the perennial herb “alyssum” Lobularia maritima (Cruciferae) stimulated a positive flight response in both sexes of Ae. Aegypti [38][32]. Moreover, acetophenone and four other compounds (1-octanol, 2-phenylethanol, benzyl cyanide, and benzyl isothiocyanate) were identified from the headspace of Lobularia maritima (L. maritima) by direct comparison with analytical standards. By testing the flight preference toward single synthetic compounds identified in the headspace of L. maritima, the reseauthorchers showed that acetophenone (1) was the first plant-derived compound that elicited positive flight behavior in Ae. aegypti (Figure 2).
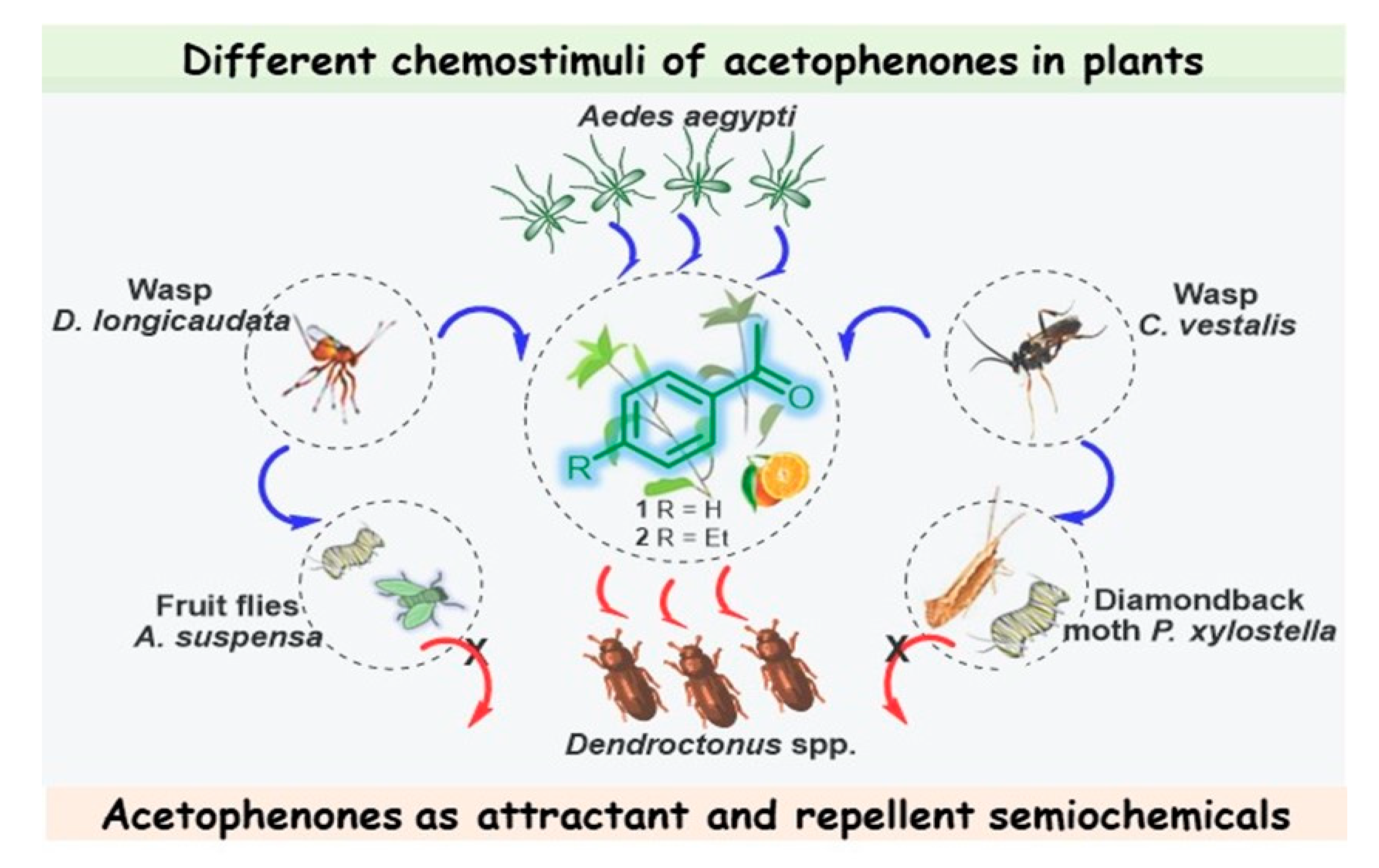
Figure 2. Different chemostimuli of acetophenones 1 and 2 in L. maritima and Citrus sinensis; multitrophic interactions between plants, herbivores, and their natural enemies, in which acetophenones act as attractant and repellent semiochemicals.
Note that this volatile compound is present in the whole plant, which is likewise attractive to the female Diachasmimorpha longicaudata wasp, which is a solitary larval endoparasitoid of Caribbean fruit flies (Anastrepha suspensa) and a major fruit pest that occurs in the New World tropics and subtropics [39][33]; however, while the males of this species can recognize ketone 1, it did not elicit a behavioral response [40][34]. Volatiles of Alyssum selectively attract and enhance the performance of another larval parasitoid (Cotesia vestalis) of the diamondback moth (Plutella xylostella), which is one of the most important pests in cruciferous plants worldwide [41][35]. Therefore, this acetophenone-containing plant, rich in nectar, which is the main sugar source for mosquitoes, could be a good candidate for use as a trap crop for these pest insects, achieving successful biological control.
Regarding coniferophagous bark beetles of the Dendroctonus genus, important damaging insect pests of pines, acetophenone (1) was identified in the volatiles of females of three species—Dendroctonus pseudotsugae (D. pseudotsugae), Dendroctonus ponderosae, and Dendroctonus rufipennis. However, it was found that acetophenone reduced only the response of female D. pseudotsugae to the attractive bait. This suggests that acetophenone (1) could serve as a repellent kairomone during host selection [42][36]. Other species, Dendroctonus frontalis [43][37] and Dendroctonus brevicomis [44[38][39][40],45,46], also produce ketone 1, described by the reseauthorchers as an antiattractant compound that inhibits the attraction of conspecifics. Interestingly, these aggressive tree-killing bark beetles can rapidly colonize their host by producing an aggregation pheromone, which can be regulated by acetophenone-based multicomponent blends. It is interesting to note that other pest beetles are addicted to acetophenone, which had a positive impact on the weight and multiplication of the red flour beetle (Tribolium castaneum), a worldwide pest of stored products, and the tobacco beetle (Lasioderma serricorne), one of the most widespread and damaging pests for the tobacco industry, when they were treated with acetophenone-containing cultures that deter the beetles from feeding well, which affected their growth, and thus, acetophenone exhibits antifeedant properties in this case [47][41]. Acetophenone is also involved in complex interactions between fungal symbionts of the ambrosia beetle Platypus cylindrus and the cork oak (Quercus suber), an interesting plant that is the primary source of cork for wine bottle stoppers. These interactions are linked to the cork oak decline process. It was found recently that strains of fungal associates of P. cylindrus, which is a major cork oak pest in Portugal, induced an increase in volatiles known for mediating ambrosia beetle behavior (mainly acetophenone, sulcatone, and nonanal) in response to fungal inoculation. This may help to elucidate the mutualistic or pathogenic aspects of these complex symbiotic interactions and develop new control strategies for P. cylindrus [48][42].
The floral scent of Antirrhinum majus pseudomajus (A. majus pseudomajus), a wild subspecies of snapdragon, also contains acetophenone as the main component, contributing over 69% of the absolute emissions of this plant. Suchet et al. showed that bumblebees (Bombus terrestris), flower visitors in the Antirrhinum genus, innately avoided A. majus pseudomajus, taking care of their fragile existence. Therefore, acetophenone (1) again turned out to be a repellent compound [49][43]. It seems that 4-ethylacetophenone (2), present as a minor constituent in the peels of navel oranges (Citrus sinensis), could also be involved in complex interrelationships with the Mediterranean fruit fly, Ceratitis capitata, eliciting a significant voltage spike in females but not in males (Figure 2) [50][44].
Moreover, acetophenone is the major component (51%) of the volatile oil of Pogostemon heyneanus (Indian patchouli) leaves [51][45], which could be useful for odor-bait technology. This oil also showed antibiofilm and antivirulence effects against Streptococcus pyogenes, an important human pathogen that causes several superficial infections and invasive diseases [52][46].
4. Biogenesis: How Can Acetophenone Be Formed In Vivo?
Acetophenone is one of the secondary metabolites and could be classified as a phenolic C6–C2 metabolite according to its basic skeleton. As mentioned previously in the Historical Background section, acetophenone is a volatile organic compound and a polar, lipophilic liquid (ε = 17.40; μ = 3.05 D; logP = 1.58) with a low molecular weight (MW = 120.15 g·mol−1) [53,54][47][48]. Its physical properties allow acetophenone to freely cross cellular membranes and be released into the surrounding environment.
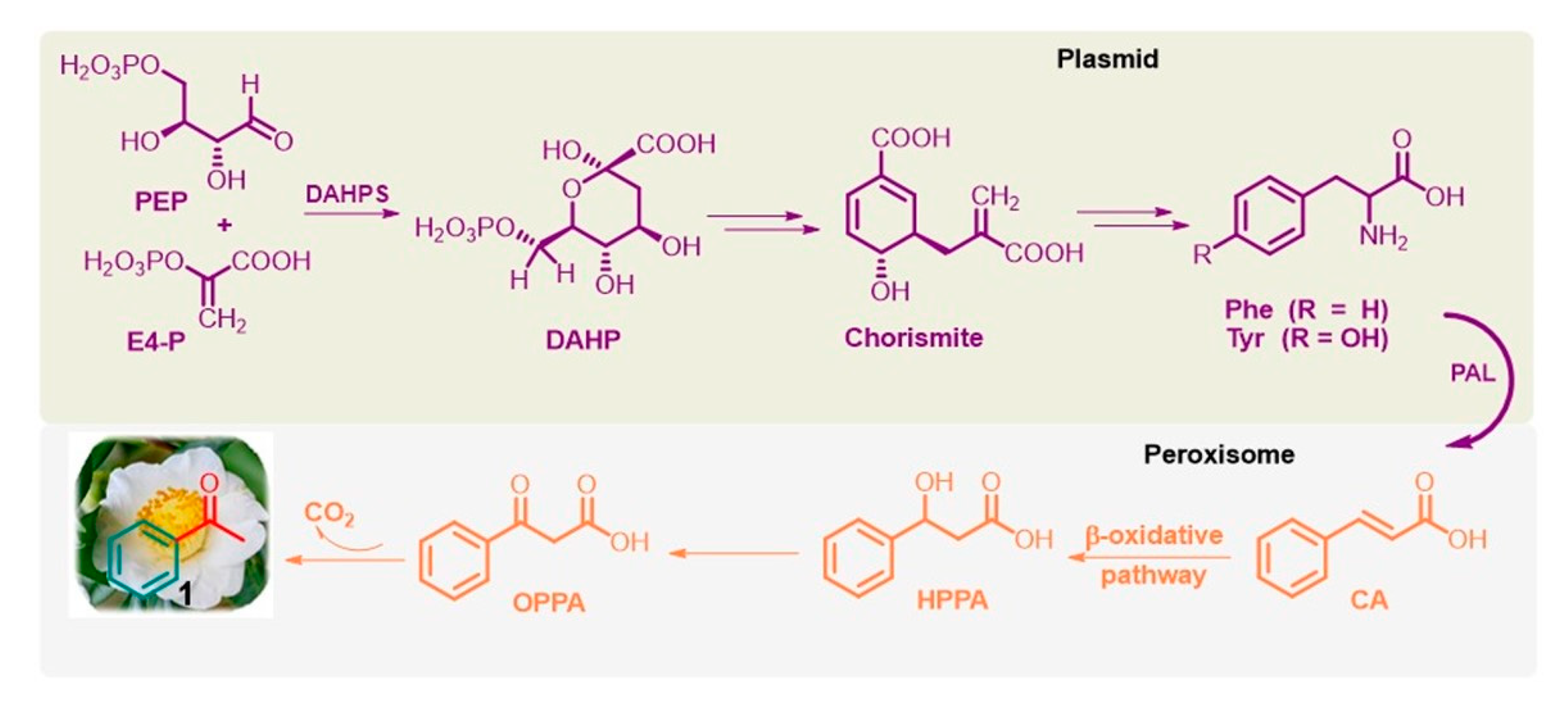
Phenolic compounds are ubiquitously present in the plant kingdom but are less common in bacteria, fungi, and algae [55][49]. Generally, plant phenolics are believed to act as defense compounds against herbivores, microbes, viruses, and compelling plants or as signal compounds to attract pollinating or repel seed-dispersing animals, mainly insects. They are mainly synthesized via the shikimate pathway [56,57][50][51], which is present in bacteria, fungi, and plants but is absent in animals. It provides phenylalanine (Phe) and tyrosine (Tyr), precursors of the corresponding cinnamic derivatives. In particular, Phe is obtained from the conversion of phosphoenolpyruvate (PEP) via the glycolysis pathway and erythrose 4-phosphate (E4-P) via the pentose phosphate pathway into chorismite via 3-deoxy-D-arabino-heptulosonate-7-phosphate (DAHP) through DAHP synthase (DAHPS; EC 2.5.1.54). Furthermore, the well-known and widely distributed enzyme L-phenylalanine ammonia lyase (PAL; EC 4.3.1.5) deaminates Phe to trans-cinnamic acid (CA) [58][52] (Figure 3).

Figure 3. Simplified shikimate pathway of L-Phe from PEP and E4-P, and the β-oxidative pathway of acetophenone (1) from CA in flowers of tea plants and fungus Bjerkandera adusta.
Notably, Phe biosynthesis occurs in plastids, and its further conversion to volatile compounds, such as acetophenone, occurs outside this organelle, especially in the peroxisome, a small membrane-enclosed oxidative vesicle. The detailed bio-origins of acetophenone are still unclear [56,57,58][50][51][52]. It is known that trans-CA can subsequently be hydroxylated to β-hydroxy-phenyl propionic acid (HPPA) and then to β-oxo-phenyl propionic acid (OPPA), which can be degraded into acetophenone. This biosynthetic pathway, called the β-oxidative pathway [59][53], was proposed for the fungus Bjerkandera adusta by Lapadatescu et al. [60][54] and explored by Dong et al. in the flowers of tea plants (C. sinensis) [61][55]. Notably, although the enzymes involved in these reactions have not yet been identified, this natural route to acetophenone is a very significant model to follow and could help develop new industrial methods for this valuable ketone based on nonpetrol sources.
References
- Clardy, J.; Walsh, C. Lessons from natural molecules. Nature 2004, 432, 829–837.
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta BBA Gen. Subj. 2013, 1830, 3670–3695.
- Pagare, S.; Bhatia, M.; Tripathi, N.; Pagare, S.; Bansal, Y.K. Secondary metabolites of plants and their role: Overview. Curr. Trends Biotechnol. Pharm. 2015, 9, 293–304.
- Khare, S.; Singh, N.B.; Singh, A.; Hussain, I.; Niharika, K.; Yadav, V.; Bano, C.; Yadav, R.K.; Amist, N. Plant secondary metabolites synthesis and their regulations under biotic and abiotic constraints. J. Plant Biol. 2020, 63, 203–216.
- Zhang, H.; Zhu, Y.; Liu, Z.; Peng, Y.; Peng, W.; Tong, L.; Wang, J.; Liu, Q.; Wang, P.; Cheng, G. A volatile from the skin microbiota of flavivirus-infected hosts promotes mosquito attractiveness. Cell 2022, 185, 2510–2522.e16.
- Leslie, M. Dengue and zika viruses turn people into mosquito bait. Science 2022, 377, 137.
- Du Toit, A. An attractive scent. Nat. Rev. Genet. 2022, 20, 510.
- Gul, L.; Korcsmaros, T.; Hall, N. Flaviviruses hijack the host microbiota to facilitate their transmission. Cell 2022, 185, 2395–2397.
- Braks, M.; Anderson, R.; Knols, B. Infochemicals in Mosquito Host Selection: Human Skin Microflora and Plasmodium Parasites. Parasitol. Today 1999, 15, 409–413.
- Verhulst, N.O.; Andriessen, R.; Groenhagen, U.; Kiss, G.B.; Schulz, S.; Takken, W.; Van Loon, J.J.A.; Schraa, G.; Smallegange, R.C. Differential Attraction of Malaria Mosquitoes to Volatile Blends Produced by Human Skin Bacteria. PLoS ONE 2010, 5, e15829.
- Verhulst, N.O.; Takken, W.; Dicke, M.; Schraa, G.; Smallegange, R.C. Chemical ecology of interactions between human skin microbiota and mosquitoes. FEMS Microbiol. Ecol. 2010, 74, 1–9.
- Verhulst, N.O.; Qiu, Y.T.; Beijleveld, H.; Maliepaard, C.; Knights, D.; Schulz, S.; Berg-Lyons, D.; Lauber, C.L.; Verduijn, W.; Haasnoot, G.W.; et al. Composition of Human Skin Microbiota Affects Attractiveness to Malaria Mosquitoes. PLoS ONE 2011, 6, e28991.
- Guerenstein, P.G.; Lazzari, C.R. Host-seeking: How triatomines acquire and make use of information to find blood. Acta Trop. 2009, 110, 148–158.
- Ortiz, M.I.; Molina, J. Preliminary evidence of Rhodnius prolixus (Hemiptera: Triatominae) attraction to human skin odour extracts. Acta Trop. 2010, 113, 174–179.
- Omolo, M.O.; Ndiege, I.O.; Hassanali, A. Semiochemical signatures associated with differential attraction of Anopheles gambiae to human feet. PLoS ONE 2021, 16, e0260149.
- Carraretto, D.; Soresinetti, L.; Rossi, I.; Malacrida, A.R.; Gasperi, G.; Gomulski, L.M. Behavioural Responses of Male Aedes albopictus to Different Volatile Chemical Compounds. Insects 2022, 13, 290.
- Fawaz, E.Y.; Allan, S.A.; Bernier, U.R.; Obenauer, P.J.; Diclaro, J.W. Swarming mechanisms in the yellow fever mosquito: Aggregation pheromones are involved in the mating behavior of Aedes aegypti. J. Vector Ecol. 2014, 39, 347–354.
- Mozūraitis, R.; Hajkazemian, M.; Zawada, J.W.; Szymczak, J.; Pålsson, K.; Sekar, V.; Biryukova, I.; Friedländer, M.R.; Koekemoer, L.L.; Baird, J.K.; et al. Male swarming aggregation pheromones increase female attraction and mating success among multiple African malaria vector mosquito species. Nat. Ecol. Evol. 2020, 4, 1395–1401.
- Torr, S.; Mangwiro, T.; Hall, D. Responses of Glossina pallidipes (Diptera: Glossinidae) to synthetic repellents in the field. Bull. Èntomol. Res. 1996, 86, 609–616.
- Olaide, O.Y.; Tchouassi, D.P.; Yusuf, A.A.; Pirk, C.W.W.; Masiga, D.K.; Saini, R.K.; Torto, B. Zebra skin odor repels the savannah tsetse fly, Glossina pallidipes (Diptera: Glossinidae). PLOS Neglected Trop. Dis. 2019, 13, e0007460.
- Kariithi, H.M.; Van Oers, M.M.; Vlak, J.M.; Vreysen, M.J.B.; Parker, A.G.; Abd-Alla, A.M.M. Virology, Epidemiology and Pathology of Glossina Hytrosavirus, and Its Control Prospects in Laboratory Colonies of the Tsetse Fly, Glossina pallidipes (Diptera; Glossinidae). Insects 2013, 4, 287–319.
- Olaide, O.Y.; Tchouassi, D.P.; Yusuf, A.A.; Pirk, C.W.; Masiga, D.K.; Saini, R.K.; Torto, B. Effect of zebra skin-derived compounds on field catches of the human African trypanosomiasis vector Glossina fuscipes fuscipes. Acta Trop. 2020, 213, 105745.
- De Moraes, C.M.; Stanczyk, N.M.; Betz, H.S.; Pulido, H.; Sim, D.G.; Read, A.F.; Mescher, M.C. Malaria-induced changes in host odors enhance mosquito attraction. Proc. Natl. Acad. Sci. USA 2014, 111, 11079–11084.
- Yamazaki, K.; Boyse, E.A.; Bard, J.; Curran, M.; Kim, D.; Ross, S.R.; Beauchamp, G.K. Presence of mouse mammary tumor virus specifically alters the body odor of mice. Proc. Natl. Acad. Sci. USA 2002, 99, 5612–5615.
- Vreysen, M.J.; Seck, M.T.; Sall, B.; Bouyer, J. Tsetse flies: Their biology and control using area-wide integrated pest management approaches. J. Invertebr. Pathol. 2012, 112, S15–S25.
- Lindh, J.M.; Torr, S.J.; Vale, G.A.; Lehane, M.J. Improving the Cost-Effectiveness of Artificial Visual Baits for Controlling the Tsetse Fly Glossina fuscipes fuscipes. PLOS Neglected Trop. Dis. 2009, 3, e474.
- Silvério, M.R.S.; Espindola, L.S.; Lopes, N.P.; Vieira, P.C. Plant Natural Products for the Control of Aedes aegypti: The Main Vector of Important Arboviruses. Molecules 2020, 25, 3484.
- Dormont, L.; Mulatier, M.; Carrasco, D.; Cohuet, A. Mosquito Attractants. J. Chem. Ecol. 2021, 47, 351–393.
- Kapsetaki, S.-E.; Tzelepis, I.; Avgousti, K.; Livadaras, I.; Garantonakis, N.; Varikou, K.; Apidianakis, Y. The bacterial metabolite 2-aminoacetophenone promotes association of pathogenic bacteria with flies. Nat. Commun. 2014, 5, 4401.
- Fikrig, K.; Johnson, B.J.; Fish, D.; Ritchie, S.A. Assessment of synthetic floral-based attractants and sugar baits to capture male and female Aedes aegypti (Diptera: Culicidae). Parasites Vectors 2017, 10, 1–9.
- Lahondère, C.; Vinauger, C.; Okubo, R.P.; Wolff, G.H.; Chan, J.K.; Akbari, O.S.; Riffell, J.A. The olfactory basis of orchid pollination by mosquitoes. Proc. Natl. Acad. Sci. USA 2019, 117, 708–716.
- von Oppen, S.; Masuh, H.; Licastro, S.; Zerba, E.; Gonzalez-Audino, P. A floral-derived attractant for Aedes aegypti mosquitoes. Èntomol. Exp. Appl. 2015, 155, 184–192.
- Ovruski, S.; Aluja, M.; Sivinski, J.; Wharton, R. Hymenopteran Parasitoids on Fruit-infesting Tephritidae (Diptera) in Latin America and the Southern United States: Diversity, Distribution, Taxonomic Status and their use in Fruit Fly Biological Control. Integr. Pest Manag. Rev. 2000, 5, 81–107.
- Rohrig, E.; Sivinski, J.; Teal, P.; Stuhl, C.; Aluja, M. A Floral-Derived Compound Attractive to the Tephritid Fruit Fly Parasitoid Diachasmimorpha longicaudata (Hymenoptera: Braconidae). J. Chem. Ecol. 2008, 34, 549–557.
- Chen, Y.; Mao, J.; Reynolds, O.L.; Chen, W.; He, W.; You, M.; Gurr, G.M. Alyssum (Lobularia maritima) selectively attracts and enhances the performance of Cotesia vestalis, a parasitoid of Plutella xylostella. Sci. Rep. 2020, 10, 6447.
- Pureswaran, D.S.; Borden, J.H. New repellent semiochemicals for three species of Dendroctonus (Coleoptera: Scolytidae). Chemoecology 2004, 14, 67–75.
- Sullivan, B.T. Electrophysiological and Behavioral Responses of Dendroctonus frontalis (Coleoptera: Curculionidae) to Volatiles Isolated from Conspecifics. J. Econ. Èntomol. 2005, 98, 2067–2078.
- Erbilgin, N.; Gillette, N.E.; Mori, S.R.; Stein, J.D.; Owen, D.R.; Wood, D.L. Acetophenone as an Anti-attractant for the Western Pine Beetle, Dendroctonus Brevicomis LeConte (Coleoptera: Scolytidae). J. Chem. Ecol. 2007, 33, 817–823.
- Erbilgin, N.; Gillette, N.E.; Owen, D.R.; Mori, S.R.; Nelson, A.S.; Uzoh, F.; Wood, D.L. Acetophenone superior to verbenone for reducing attraction of western pine beetle Dendroctonus brevicomis to its aggregation pheromone. Agric. For. Entomol. 2008, 10, 433–441.
- Fettig, C.J.; McKelvey, S.R.; Dabney, C.P.; Huber, D.P.W. Responses of Dendroctonus brevicomis (Coleoptera: Curculionidae) in behavioral assays: Implications to development of a semiochemical-based tool for tree protection. J. Econ. Èntomol. 2012, 105, 149–160.
- Jonfia-Ess, W.; Alderson, P.; Tucker, G.; Linforth, R.; West, G. The Growth of Tribolium castaneum (Herbst) and Lasioderma serricorne (Fabricius) on Feed Media Dosed with Flavour Volatiles Found in Dry Cocoa Beans. Pak. J. Biol. Sci. 2007, 10, 1301–1304.
- Nones, S.; Sousa, E.; Holighaus, G. Symbiotic fungi of an ambrosia beetle alter the volatile bouquet of cork oak seedlings. Phytopathology 2022, 112, 1965–1978.
- Suchet, C.; Dormont, L.; Schatz, B.; Giurfa, M.; Simon, V.; Raynaud, C.; Chave, J. Floral scent variation in two Antirrhinum majus subspecies influences the choice of naïve bumblebees. Behav. Ecol. Sociobiol. 2011, 65, 1015–1027.
- Hernández, M.M.; Sanz, I.; Adelantado, M.; Ballach, S.; Primo, E. Electroantennogram activity from antennae ofCeratitis capitata (Wied.) to fresh orange airborne volatiles. J. Chem. Ecol. 1996, 22, 1607–1619.
- Murugan, R.; Mallavarapu, G.R.; Padmashree, K.V.; Rao, R.R.; Livingstone, C. Volatile Oil Composition of Pogostemon heyneanus and Comparison of its Composition with Patchouli Oil. Nat. Prod. Commun. 2010, 5, 1961–1964.
- Nithyanand, P.; Shafreen, R.M.B.; Muthamil, S.; Murugan, R.; Pandian, S.K. Essential oils from commercial and wild Patchouli modulate Group A Streptococcal biofilms. Ind. Crop. Prod. 2015, 69, 180–186.
- Bodor, N.; Gabanyi, Z.; Wong, C.K. A new method for the estimation of partition coefficient. J. Am. Chem. Soc. 1989, 111, 3783–3786.
- Acetophenone. Available online: http://www.stenutz.eu/chem/solv6.php?name=acetophenone (accessed on 29 July 2022).
- Marchiosi, R.; Dos Santos, W.D.; Constantin, R.P.; De Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Mota, T.R.; de Oliveira, D.M.; Foletto-Felipe, M.D.P.; Abrahão, J.; et al. Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem. Rev. 2020, 19, 865–906.
- Lattanzio, V. Relationship of Phenolic Metabolism to Growth in Plant and Cell Cultures Under Stress. In Plant Cell and Tissue Differentiation and Secondary Metabolites. Reference Series in Phytochemistry; Ramawat, K.G., Ekiert, H.M., Goyal, S., Eds.; Springer: Cham, Switzerland, 2021; pp. 837–868.
- Widhalm, J.R.; Dudareva, N. A Familiar Ring to It: Biosynthesis of Plant Benzoic Acids. Mol. Plant 2015, 8, 83–97.
- Maeda, H.; Dudareva, N. The Shikimate Pathway and Aromatic Amino Acid Biosynthesis in Plants. Annu. Rev. Plant Biol. 2012, 63, 73–105.
- Qualley, A.V.; Widhalm, J.R.; Adebesin, F.; Kish, C.M.; Dudareva, N. Completion of the core β-oxidative pathway of benzoic acid biosynthesis in plants. Proc. Natl. Acad. Sci. USA 2012, 109, 16383–16388.
- Lapadatescu, C.; Giniès, C.; Le Quéré, J.-L.; Bonnarme, P. Novel Scheme for Biosynthesis of Aryl Metabolites from l -Phenylalanine in the Fungus Bjerkandera adusta. Appl. Environ. Microbiol. 2000, 66, 1517–1522.
- Dong, F.; Yang, Z.; Baldermann, S.; Kajitani, Y.; Ota, S.; Kasuga, H.; Imazeki, Y.; Ohnishi, T.; Watanabe, N. Characterization of l-phenylalanine metabolism to acetophenone and 1-phenylethanol in the flowers of Camellia sinensis using stable isotope labeling. J. Plant Physiol. 2011, 169, 217–225.
More
