Hypertension is defined as repeated elevated office systolic blood pressure (SBP) values over 140 mmHg and/or diastolic BP (DBP) over 90 mmHg or average home BP over 135/85 mmHg. Metabolic syndrome (MetS) has serious outcomes regarding the individual’s health, with increasing prevalence nowadays and a significant impact on healthcare systems. Its definition varied over time. MetS consists of several conditions, such as hypertension, elevated fasting glucose (over 100 mg/dL) or type 2 diabetes mellitus (T2DM), decreased high-density lipoprotein cholesterol levels (less than 40 mg/dL in men or 50 mg/dL in women), high triglycerides concentrations (over 150 mg/dL) and waist circumference over 40 inches (men) or 35 inches (women).
- hypertension
- metabolic syndrome
- type 2 diabetes mellitus
1. Introduction
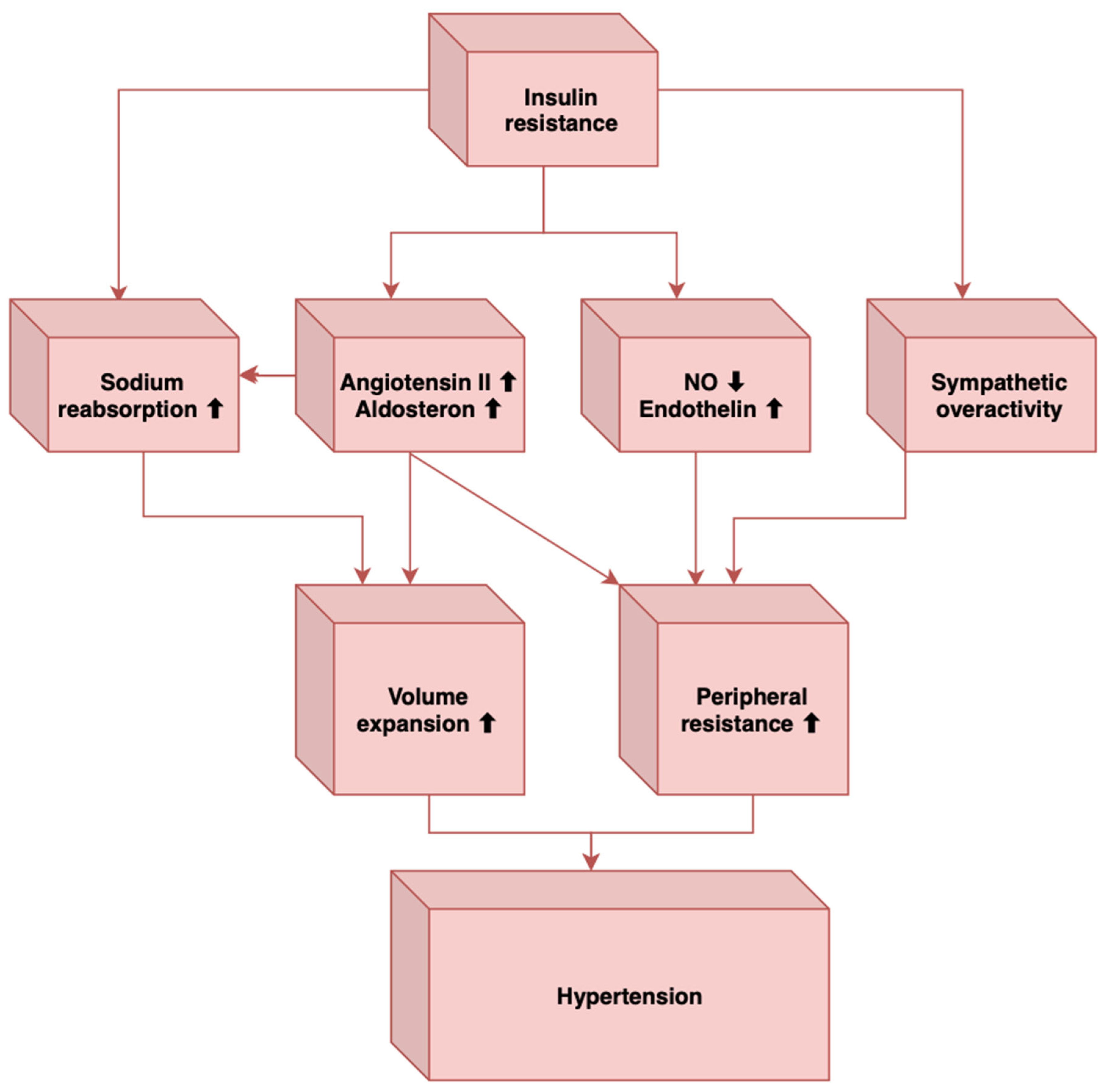
2. Pathogenesis of Hypertension in MetS
3. Links between Hypertension and Metabolic Syndrome
References
- Franklin, S.S. Hypertension in the Metabolic Syndrome. Metab. Syndr. Relat. Disord. 2006, 4, 287–298.
- Bergler-Klein, J. What’s New in the ESC 2018 Guidelines for Arterial Hypertension: The Ten Most Important Messages. Wien. Klin. Wochenschr. 2019, 131, 180–185.
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the Management of Arterial Hypertension. Eur. Heart J. 2018, 39, 3021–3104.
- Swarup, S.; Goyal, A.; Grigorova, Y.; Zeltser, R. Metabolic Syndrome; StatPearls: Treasure Island, FL, USA, 2022.
- Bovolini, A.; Garcia, J.; Andrade, M.A.; Duarte, J.A. Metabolic Syndrome Pathophysiology and Predisposing Factors. Int. J. Sports Med. 2021, 42, 199–214.
- Fahed, C.; Aoun, L.; Zerdan, M.B.; Allam, S.; Zerdan, M.B.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786.
- Reaven, G.M. Banting Lecture 1988. Role of Insulin Resistance in Human Disease. Diabetes 1988, 37, 1595–1607.
- Kemp, H.G. Left Ventricular Function in Patients with the Anginal Syndrome and Normal Coronary Arteriograms. Am. J. Cardiol. 1973, 32, 375–376.
- Cheng, T.O. Cardiac Syndrome X versus Metabolic Syndrome X. Int. J. Cardiol. 2007, 119, 137–138.
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic syndrome: Pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225.
- NCBI. Available online: https://www.ncbi.nlm.nih.gov/books/NBK513253 (accessed on 21 December 2022).
- Regufe, V.M.G.; Pinto, C.M.C.B.; Perez, P.M.V.H.C. Metabolic syndrome in type 2 diabetic patients: A review of current evidence. Porto Biomed. J. 2020, 5, e101.
- Jambi, H.; Enani, S.; Malibary, M.; Bahijri, S.; Eldakhakhny, B.; Al-Ahmadi, J.; Al Raddadi, R.; Ajabnoor, G.; Boraie, A.; Tuomilehto, J. The Association Between Dietary Habits and Other Lifestyle Indicators and Dysglycemia in Saudi Adults Free of Previous Diagnosis of Diabetes. Nutr. Metab. Insights 2020, 15, 1178638820965258.
- Jang, H.; Park, K. Omega-3 and omega-6 polyunsaturated fatty acids and metabolic syndrome: A systemic review and meta-analysis. Clin. Nutr. 2020, 39, 765–773.
- Onat, A.; Hergenc, G.; Sari, I.; Turkmen, S.; Can, G.; Sansoy, V. Dyslipidemic hypertension: Distinctive features and cardiovascular risk in a prospective population-based study. Am. J. Hypertens. 2005, 18, 409–416.
- Garcia-Puig, J.; Ruilope, L.M.; Luque, M.; Fernandez, J.; Ortega, R.; Dal-Re, R. Glucose metabolism in patients with essential hypertension. Am. J. Med. 2006, 119, 318–326.
- Chen, W.; Li, F.; He, C.; Zhu, Y.; Tan, W. Elevated prevalence of abdominal glucose metabolism in patients with primary aldosteronism: A meta-analysis. Ir. J. Med. Sci. 2014, 183, 283–291.
- Fathi Dizaji, B. The Investigations of Genetic Determinants of the Metabolic Syndrome. Diabetes Metab. Syndr. 2018, 12, 783–789.
- Stamler, J.; Rhomberg, P.; Schoenberger, J.A.; Shekelle, R.B.; Dyer, A.; Shekelle, S.; Stamler, R.; Wannamaker, J. Multivariate Analysis of the Relationship of Seven Variables to Blood Pressure: Findings of the Chicago Heart Association Detection Project in Industry, 1967–1972. J. Chronic. Dis. 1975, 28, 527–548.
- Florey, C.V.; Uppal, S.; Lowy, C. Relation between Blood Pressure, Weight, and Plasma Sugar and Serum Insulin Levels in Schoolchildren Aged 9–12 Years in Westland, Holland. Br. Med. J. 1976, 1, 1368–1371.
- Jarrett, R.J.; Keen, H.; Mccartney, M.; Fuller, J.H.; Hamilton, P.J.S.; Reid, D.D.; Rose, G. Glucose Tolerance and Blood Pressure in Two Population Samples: Their Relation to Diabetes Mellitus and Hypertension. Int. J. Epidemiol. 1978, 7, 15–24.
- Persky, V.; Dyer, A.; Stamler, J.; Shekelle, R.B.; Schoenberger, J.; Wannamaker, J.; Upton, M. The Relationship between Post-Load Plasma Glucose and Blood Pressure at Different Resting Heart Rates. J. Chronic. Dis. 1979, 32, 263–268.
- Voors, A.W.; Radhakrishnamurthy, B.; Srinivasan, S.R.; Webber, L.S.; Berenson, G.S. Plasma Glucose Level Related to Blood Pressure in 272 Children, Ages 7-15 Years, Sampled from a Total Biracial Population. Am. J. Epidemiol. 1981, 113, 347–356.
- Lucas, C.P.; Estigarribia, J.A.; Darga, L.L.; Reaven, G.M. Insulin and Blood Pressure in Obesity. Hypertension 1985, 7, 702–706.
- Singer, P.; Gödicke, W.; Voigt, S.; Hajdu, I.; Weiss, M. Postprandial Hyperinsulinemia in Patients with Mild Essential Hypertension. Hypertension 1985, 7, 182–186.
- Modan, M.; Halkin, H.; Almog, S.; Lusky, A.; Eshkol, A.; Shefi, M.; Shitrit, A.; Fuchs, Z. Hyperinsulinemia. A Link between Hypertension Obesity and Glucose Intolerance. J. Clin. Investig. 1985, 75, 809–817.
- Manicardi, V.; Camellini, L.; Bellodi, G.; Coscelli, C.; Ferrannini, E. Evidence for an Association of High Blood Pressure and Hyperinsulinemia in Obese Man. J. Clin. Endocrinol. Metab. 1986, 62, 1302–1304.
- Rowe, J.W.; Young, J.B.; Minaker, K.L.; Stevens, A.L.; Pallotta, J.; Landsberg, L. Effect of Insulin and Glucose Infusions on Sympathetic Nervous System Activity in Normal Man. Diabetes 1981, 30, 219–225.
- Christensen, N.J.; Gundersen, H.J.G.; Hegedüs, L.; Jacobsen, F.; Mogensen, C.E.; Østerby, R.; Vittinghus, E. Acute Effects of Insulin on Plasma Noradrenaline and the Cardiovascular System. Metabolism 1980, 29 (Suppl. 1), 1138–1145.
- Baum, M. Insulin Stimulates Volume Absorption in the Rabbit Proximal Convoluted Tubule. J. Clin. Investig. 1987, 79, 1104–1109.
- Landsberg, L.; Young, J.B. Diet and the Sympathetic Nervous System: Relationship to Hypertension. Int. J. Obes. 1981, 5, 79–91.
- Hwang, I.S.; Ho, H.; Hoffman, B.B.; Reaven, G.M. Fructose-Induced Insulin Resistance and Hypertension in Rats. Hypertension 1987, 10, 512–516.
- Romero-Nava, R.; García, N.; Aguayo-Cerón, K.A.; Sánchez Muñoz, F.; Huang, F.; Hong, E.; Villafaña, S. Modifications in GPR21 and GPR82 Genes Expression as a Consequence of Metabolic Syndrome Etiology. J. Recept. Signal Transduct. Res. 2021, 41, 38–44.
- Ionescu, R.F.; Enache, R.M.; Cretoiu, S.M.; Cretoiu, D. The Interplay Between Gut Microbiota and MiRNAs in Cardiovascular Diseases. Front. Cardiovasc. Med. 2022, 9, 856901.
- Gharipour, M.; Sadeghi, M. Pivotal Role of MicroRNA-33 in Metabolic Syndrome: A Systematic Review. ARYA Atheroscler. 2013, 9, 372.
- Wang, Y.T.; Tsai, P.C.; Liao, Y.C.; Hsu, C.Y.; Juo, S.H.H. Circulating MicroRNAs Have a Sex-Specific Association with Metabolic Syndrome. J. Biomed. Sci. 2013, 20, 72.
- Tripathy, D.; Mohanty, P.; Dhindsa, S.; Syed, T.; Ghanim, H.; Aliada, A.; Dandona, P. Elevation of Free Fatty Acids Induces Inflammation and Impairs Vascular Reactivity in Healthy Subjects. Diabetes 2003, 52, 2882–2887.
- Lent-Schochet, D.; McLaughlin, M.; Ramakrishnan, N.; Jialal, I. Exploratory Metabolomics of Metabolic Syndrome: A Status Report. World J. Diabetes 2019, 10, 23–36.
- Yamada, J.; Tomiyama, H.; Yambe, M.; Koji, Y.; Motobe, K.; Shiina, K.; Yamamoto, Y.; Yamashina, A. Elevated Serum Levels of Alanine Aminotransferase and Gamma Glutamyltransferase Are Markers of Inflammation and Oxidative Stress Independent of the Metabolic Syndrome. Atherosclerosis 2006, 189, 198–205.
- Velasquez, M.T.; Ramezani, A.; Manal, A.; Raj, D.S. Trimethylamine N-Oxide: The Good, the Bad and the Unknown. Toxins 2016, 8, 326.
- Tang, W.H.W.; Hazen, S.L. Microbiome, Trimethylamine N-Oxide, and Cardiometabolic Disease. Transl. Res. 2017, 179, 108–115.
- Hart, L.M.T.; Vogelzangs, N.; Mook-Kanamori, D.O.; Brahimaj, A.; Nano, J.; van der Heijden, A.A.W.A.; van Dijk, K.W.; Slieker, R.C.; Steyerberg, E.W.; Ikram, A.; et al. Blood Metabolomic Measures Associate With Present and Future Glycemic Control in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2018, 103, 4569–4579.
- Adams, S.H. Emerging Perspectives on Essential Amino Acid Metabolism in Obesity and the Insulin-Resistant State. Adv. Nutr. 2011, 2, 445–456.
- Chen, T.; Zheng, X.; Ma, X.; Bao, Y.; Ni, Y.; Hu, C.; Rajani, C.; Huang, F.; Zhao, A.; Jiia, W.; et al. Tryptophan Predicts the Risk for Future Type 2 Diabetes. PLoS ONE 2016, 11, e0162192.
- Oxenkrug, G.; van der Hart, M.; Summergrad, P. Elevated Anthranilic Acid Plasma Concentrations in Type 1 but Not Type 2 Diabetes Mellitus. Integr. Mol. Med. 2015, 2, 365–368.
- Yanai, H.; Tomono, Y.; Ito, K.; Furutani, N.; Yoshida, H.; Tada, N. The Underlying Mechanisms for Development of Hypertension in the Metabolic Syndrome. Nutr. J. 2008, 7, 10.
- Manrique, C.; Lastra, G.; Sowers, J.R. New Insights into Insulin Action and Resistance in the Vasculature. Ann. N. Y. Acad. Sci. 2014, 1311, 138–150.
- Mendizábal, Y.; Llorens, S.; Nava, E. Hypertension in Metabolic Syndrome: Vascular Pathophysiology. Int. J. Hypertens. 2013, 2013, 230868.
- Alvarez, G.E.; Beske, S.D.; Ballard, T.P.; Davy, K.P. Sympathetic Neural Activation in Visceral Obesity. Circulation 2002, 106, 2533–2536.
- de Jongh, R.T.; Serné, E.H.; Ijzerman, R.G.; de Vries, G.; Stehouwer, C.D.A. Impaired Microvascular Function in Obesity: Implications for Obesity-Associated Microangiopathy, Hypertension, and Insulin Resistance. Circulation 2004, 109, 2529–2535.
- Kirpichnikov, D.; Sowers, J.R. Diabetes Mellitus and Diabetes-Associated Vascular Disease. Trends Endocrinol. Metab. 2001, 12, 225–230.
- Umeda, M.; Kanda, T.; Murakami, M. Effects of Angiotensin II Receptor Antagonists on Insulin Resistance Syndrome and Leptin in Sucrose-Fed Spontaneously Hypertensive Rats. Hypertens. Res. 2003, 26, 485–492.