Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Yamir Jiménez-Viveros and Version 2 by Rita Xu.
Capsicum
is one of the most economically important genera in the Solanaceae family.
Capsicum
fruits (peppers) are rich in phytochemicals with high nutritional value and significant health-promoting characteristics. The phytochemical profile of peppers consists of capsaicinoids, carotenoids, and phenolics, primarily.
- capsaicinoids
- carotenoids
- irradiance
- phenolic compounds
- plant secondary metabolites
1. Introduction
Capsicum is one of the most economically important genera in the Solanaceae family. This genus encompasses five domesticated species with more than 50,000 cultivars [1]. The fruits of Capsicum (peppers) are associated with significant health-promoting properties attributable to their nutritional composition and metabolite contents. These properties include analgesic, anti-obesity, cardioprotective, pharmacological, neurological, and dietetic, among others [2]. The specific phytochemicals associated with these properties include carotenoids (provitamin A), phenolic compounds, and capsaicinoids, primarily [3].
The phytochemical and secondary metabolite profiles of peppers are also a good source of nutrients and bioactive compounds [4][5][4,5]. Secondary metabolites are a large group of organic compounds with low molecular weight and specific physiological functions. These metabolites serve as chemical adaptations to stress conditions, or as defensive, protective, or offensive chemical agents against micro-organisms, insects, and herbivores [6].
The chemical composition of peppers is closely related to genotype, the process of fruit ripening [3][7][3,7], and environmental conditions [8][9][8,9]. The environmental factors that affect the biosynthesis, metabolism, and accumulation of phytochemicals in peppers include light, temperature, soil-water availability, and plant nutrition [10]. Thus, changes in environmental conditions can affect the biosynthesis of bioactive compounds in peppers [8].
Peppers vary in color, shape, and chemical composition [7]. Color properties vary by genotype and cultivar. Color changes occur during fruit maturation when the plastids transition from chloroplast to chromoplast in the fruits’ pericarp [3].
Currently, the production of peppers is carried out predominantly under protected horticulture conditions [11]. In particular, the manipulation of natural light by photo-selective netting or plastics, and supplemental lighting (artificial light) can be used to reduce heat and light stress and improve the yield and quality of horticultural crops [12]. These horticultural practices modify the light intensity and spectrum intercepted by the plants and may also affect the production levels of total phenols, ascorbic acid, and antioxidants due to the influence of modified light conditions on the metabolic pathways that lead to the formation of the phytochemicals [13]. Controlled growing conditions in glasshouses impacted the carotenoid contents in sweet peppers [14]. Thus, light intensity (irradiance) and spectrum are environmental factors that affect the phytochemical contents of peppers [15].
2. Light Interactions with Capsicum Plants
The growth and productivity of pepper crops are affected by environmental factors [16]. Among these factors, light is the principal source of energy that drives physiological processes, which include: photosynthesis, photomorphogenesis, fruit development, and maturation [17][18][17,18]. Plants interact with light through specific pigments that acquire light energy, and photoreceptors which are proteins that elicit different responses based on light conditions [19]. The most important plant photoreceptors reported for pepper plants include phytochromes, cryptochromes, phototropins, and UV-B-Resistance 8 (UVR8) photoreceptors (Figure 1) [20]. These photoreceptors have peak absorbance wavelengths for the induction of the responses.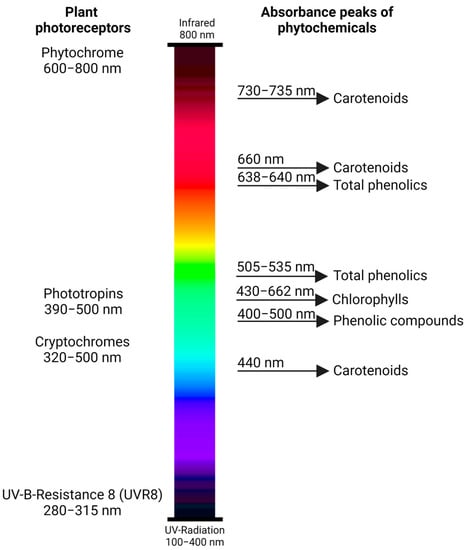
Figure 1. Plant photoreceptors (phytochrome, phototropins, cryptochromes, and UV-B-Resistance 8 (UVR8)) with the corresponding absorbance peaks (wavelengths of the electromagnetic spectrum) for each light-sensing photoreceptor protein. The light-responding groups of phytochemicals in plants in the specific wavelength ranges are provided on the right.
3. Effects of Light Characteristics on the Phytochemicals of Capsicum Fruits
The most abundant secondary metabolites in Capsicum fruits include capsaicinoids, carotenoids, phenolic compounds, flavonoids, and a wide range of volatile compounds. The accumulation of phytochemicals in peppers is light-dependent, and the high variability of these compounds determines the diversity of aroma and flavor of peppers [40].3.1. Capsaicinoids
Capsaicinoids are secondary metabolites biosynthesized exclusively by the fruits of Capsicum plants [41]. These metabolites are the bioactive compounds responsible for the pungent taste of peppers [42]. Capsaicinoids may occur in peppers in a wide range of contents from ‘Bell peppers’, where they are practically non-existent, to other high-pungency cultivars such as ‘Naga peppers’ [43]. Capsaicinoids are considered natural defense mechanisms against herbivores ranging from insects to rodents [1]. Capsaicinoids also mediate interactions with birds, who act as seed dispersers for wild peppers [44]. In recent years, capsaicinoid research has been influential in the development of innovative applications in the food and pharmaceutical industries [41] due to their value as antioxidants (free radical scavengers) [45], anti-arthritic [46], gastroprotective [47][48][47,48], anti-cancer [49], and analgesic agents [50], among others. The most abundant capsaicinoids in peppers are capsaicin and dihydrocapsaicin [51][52][51,52]. Together, these compounds encompass more than 90% of the total capsaicinoid content of peppers [53]. Nonetheless, at least nine other capsaicinoids including nordihydrocapsaicin, homodihydrocapsaicin, and homocapsaicin have also been identified [43]. Capsaicinoid levels are influenced by the ontogenetic development of the peppers. The accumulation of capsaicinoids starts at the early stages of fruit development, followed by a high peak and a rapid decline [54].3.1.1. Biosynthesis of Capsaicinoids
Capsaicinoid biosynthesis is derived from the phenylpropanoid pathway (Figure 2) [54][55][56][54,55,56] and occurs after the enzymatic condensation of a molecule of vanillylamine derived from phenylalanine, valine, or leucine to a branched-chain amino acid. The enzymes whose alleles determine pungency levels in peppers are CaMYB31, pAMT, CS/AT3/Pun1, and CaKR1 [57]. Capsaicin synthase (CS) is the last enzyme (encoded by the Pun1 gen) responsible for the condensation between vanillylamine and a fatty acid-CoA while the aromatic vanillylamine moiety is paired with many acyl groups, mostly medium-length (from 9 to 11 carbon atoms), giving the immediate reaction of capsaicin biosynthesis [58][59][58,59]. Capsaicinoids differ in their chemical structures, specifically in the side chain with a variable number of double bonds placed in different positions; the type of capsaicinoid depends on the products obtained from the different fatty acids in the dehydration synthesis reaction [55].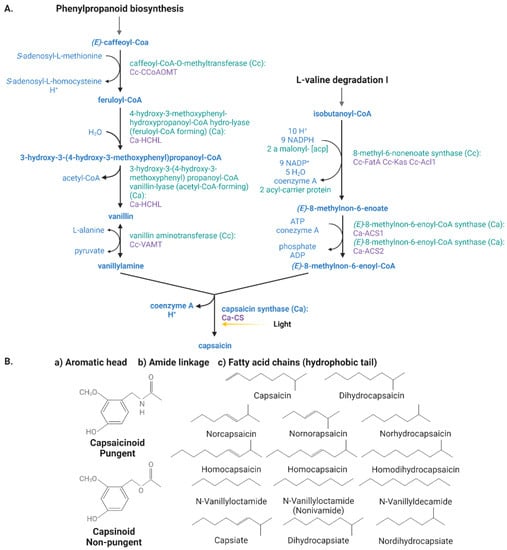
Figure 2. (A) Capsaicinoid biosynthetic pathway in peppers (Capsicum spp.) via phenylpropanoid and L-valine Degradation I. The yellow arrow indicates the light signal that regulates transcription factors at the molecular level. (B) Chemical structure of the most abundant capsaicinoids (pungent) and capsinoids (non-pungent) molecules of Capsicum fruits. Capsaicinoids and capsinoids differ in the R group (fatty acids) present.
3.1.2. Effects of Light on Capsaicinoids
In a study on bell pepper production, the optimum light intensity reported to obtain maximum fruit yield was estimated in the range of 1365 to 1470 µmol·m−2·s−1 [62]. Horticultural practices that modify irradiance may result in the enhancement or reduction of capsaicinoid contents (Table 1), depending on the species and the light modification mechanisms (e.g., color and degree of shading, or quality of light emitted by artificial illumination) [63]. Capsaicinoid accumulation is affected by the interaction of light intensity with temperature and relative humidity. In high-pungency peppers (C. chinense Jacq.), reduced light intensity and temperature caused lower capsaicinoid production of 4.82 and 3.49 mg plant−1 when plants were grown under 50% and 70% shade, respectively [63]. Reduced capsaicinoid accumulation also occurred at high irradiance levels and high temperatures. In addition, environments with reduced light intensity (713–783 µmol·m−2·s−1) and higher relative humidity increased capsaicinoid production [64]. Thus, the researcheuthors suggest an optimum light intensity of 700 to 950 µmol·m−2·s−1 for capsaicinoid production in these cultivars [63]. Total capsaicinoid contents were significantly affected by the interaction of reduced light intensity using different color shades and harvest time in C. annuum ‘Star flame’ and ‘Fire flame’ [65]. The capsaicinoid contents of peppers grown under colored shading net treatments (white, red, and green) were higher than the unshaded treatment. Of those, the green shade treatment had a considerably higher capsaicinoid content at the first harvest time. This effect could be related to a higher average temperature (22–28 °C) during the cycle. However, other studies showed that higher average temperature and increased solar radiation were associated with lower capsaicinoid contents [41]. Exposure of pepper plants (C. chinense Jacq.) to reduced light intensities using shade nets increased the contents of secondary metabolites, including capsaicinoids and other phenolic compounds [63]. Reduced light intensities increased the contents of the phenylalanine ammonia-lyase (PAL) enzyme, which plays a vital role in capsaicinoid biosynthesis. Thus, an increase in the contents of PAL may also cause an increase in capsaicinoids in peppers [66]. Currently, there is not a full understanding of how capsaicinoid accumulation relates to the relevant biochemical reactions with precursors and environmental factors [58]. As for supplemental light, pepper fruits accumulated more capsaicinoids in plants grown in a closed environment under continuous fluorescent illumination (150–350 µmol·m−2·s−1) and constant temperature (28 °C) than pepper fruits grown under greenhouse conditions during the summer season [67].Table 1. Effect of light condition treatments on the capsaicinoid content in Capsicum species.
| Capsicum | spp. | Light Treatment | Effects on Capsaicinoids Compared to Control |
Biosynthetic Effect | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C. chinense | Jacq. Seven hot hybrid peppers | Light intensities (1200, 1313, 713, 1112, 774, and 783 μmol·m | −2 | ·s | −1 | ) in different locations with shading net with 50% shade | Reduced light intensity (713–783 µmol·m | −2 | ·s | −1 | ) and higher relative humidity increased capsaicinoid production in cultivars | Not reported [64] | ||||||
| C. chinense | Jacq. ‘Bhut Jolokia’ ‘Akanee Pirote’ ‘Habanero’ |
Shading nets with 50%, and 70% shade, and unshaded as control | ‘Bhut Jolokia’ showed the highest capsaicinoid yield under 70% shading, ‘Akanee Pirote’ under 50% shading, and habanero peppers showed the lowest capsaicinoid content under shading treatments | Levels of phenylalanine ammonia-lyase (PAL) increased under low light intensities [63] | ||||||||||||||
| C. annuum | ‘Star flame’ ‘Fire flame’ |
Colored shading nets: white, red, and green with 40% shade, and unshaded as control | Capsaicinoid content increased in color-shading treatments, specifically in green treatment in both cultivars | A high average temperature of 22–28 °C may have promoted capsaicinoid biosynthesis [65] | ||||||||||||||
| C. annuum | ‘Super hot’ |
Greenhouse conditions with LED lighting treatments: blue, red, and a mixture of blue and red light, and 12 h of sunlight as control | Blue LEDs significantly increased nordihydrocapsaicin, capsaicin, dihydrocapsaicin, homocapsaicin, and homodihydrocapsaicin contents by 57, 43, 56, 28, and 54%, respectively | Capsaicin and dihydrocapsaicin accumulation helped in oxidative stress defense. Valine and phenylalanine increased in blue LED lights contributing to a higher content of capsaicinoids [68] | ||||||||||||||
| C. annuum | ‘Cheonyang’ | LED lighting treatments: red, blue, and red plus blue, and fluorescent lamps as control |
Blue LEDs increased capsaicinoid contents, red LEDs reduce two times the capsaicinoid content compared to fluorescent light | Not reported [36] | ||||||||||||||
| C. annuum | ‘Shishito pepper’ |
Continuous fluorescent illumination (150–350 µmol·m | −2 | ·s | −1 | ) at constant temperature (28 °C), and greenhouse conditions as control | Fewer seeds and higher concentration of capsaicin in fruits under continuous fluorescent illumination | There is a negative correlation between seed formation and capsaicin biosynthesis [67] | ||||||||||
| C. annuum | Serrano ‘Tampiqueño 74′ Sweet pepper ‘California wonder’ |
Artificial light in postharvest (50 µmol·m | −2 | ·s | −2 | ) and dark conditions as control | Light factors increased capsaicin content in ‘Tampiqueño 74′ | CaMYB31-expression analysis from placental tissue of pungent and non-pungent fruits showed a positive correlation with the structural genes | Ca4H | , | Comt | , | KAS | , | pAMT | , and | AT3 | expression, and with the content of capsaicin and dihydrocapsaicin during fruit development [60] |
3.2. Carotenoids
Carotenoids are a numerous family of more than 850 naturally occurring lipophilic isoprenoid compounds widely distributed in nature [69]. All photosynthetic organisms, including plants, algae, and cyanobacteria, and some non-photosynthetic micro-organisms, including fungi and bacteria, synthesize carotenoids [70]. In plants, the principal function of carotenoids is the protection of cells and organelles against oxidative damage. Carotenoids prevent the accumulation of harmful oxygen species by interacting with singlet oxygen molecules and scavenging peroxy radicals [71]. Carotenoids are also involved in the photosynthetic process and play a role in photo-protection, photo-morphogenesis, and plant development. Carotenoids also promote the biosynthesis of other essential compounds and play a role in the attraction of insects for pollination and seed dispersal [4][71][72][4,71,72]. Carotenoids have several important essential functions in human nutrition and health. This group of compounds can prevent and protect from cardiovascular diseases, inhibit carcinogenic cells, macular degeneration, and cataracts [73]. Carotenoids are considered the most effective antioxidant compounds found in peppers, besides phenolic and flavonoid compounds, which act synergistically as efficient free radical scavengers [74][75][74,75]. Carotenoids deactivate free radicals and quench reactive oxygen species due to the presence of conjugated double bonds [42][76][42,76]. In addition, plant carotenoids are endogenous isoprenoid precursors of vitamin A, β-carotene, α-carotene, γ-carotene, and β-cryptoxanthin which can be converted into retinol, the assimilable form of vitamin A in the human body [77]. Capsicum fruits are rich sources of carotenoids. The wide range of colors in peppers is related to the stage of maturation and the differential accumulation of carotenoids [78][79][78,79]. Specifically, oxygenated carotenoids are responsible for the yellow, orange, and red colors of pepper fruits [80].3.2.1. Biosynthesis of Carotenoids
Carotenoids are derived from the universal five-carbon precursor isopentenyl pyrophosphate (IPP, C5) [7]. In Capsicum, the plastidial isoprenoid biosynthesis pathway starts with the mevalonic acid which is entered into several reactions to produce the C5 building block precursors—isopentenyl diphosphate and dimethylallyl pyrophosphate. In plants, carotenoids are synthesized in the plastid using IPP generated from the methylerythritol-4-phosphate (MEP) pathway (Figure 3) [4][81][4,81]. The MEP pathway receives substrates, G3P and pyruvate, from primary metabolism and delivers IPP to the prenyl lipid pathway. Phytoene, the first carotenoid in the pathway, is synthesized from eight IPP units in the prenyl lipid pathway [72]. The carotenoid biosynthesis pathway is split into the α and β branches. The addition of a hydroxyl group to the end rings characterizes the transition from carotene to xanthophyll. The end-products found in red Capsicum fruits are the red pigments capsorubin and capsanthin with κ end groups, the latter being the most abundant [7].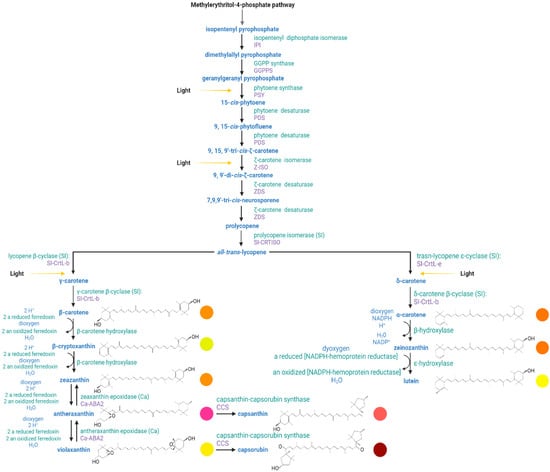
Figure 3. Carotenoid biosynthetic pathway in peppers (Capsicum spp.). Yellow arrows indicate the specific reaction steps at which light signal regulates the transcription factors at the molecular level. Chemical structures of the most abundant carotenoids present in Capsicum fruits. The circles indicate the color to which each carotenoid is associated in plant tissue.
