Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Conner Chen and Version 2 by Conner Chen.
Bacterial proteases participate in the proteolytic elimination of misfolded or aggregated proteins, carried out by members of the AAA+ protein superfamily such as Hsp100/Clp (heat shock protein-100/caseinolytic protease), Lon, and FtsH. It is estimated that the Clp and Lon families perform around 80% of cellular proteolysis in bacteria. The HSP100/Clp family of ATPases plays crucial roles in the folding, assembly, and degradation of proteins during normal growth and, mainly, under stress-inducing conditions. This family is formed by several ATPase chaperones and the peptidase ClpP (caseinolytic protease proteolytic subunit).
- Clp proteases
- Gram-positive
- chaperone-protease complex
1. Introduction
Proteolysis mediates the selective renewal of several cellular proteins, eliminating those that are defective or unwanted, thus allowing quality control of proteins and the different cellular processes [1]. One of the functions of bacterial proteases is the proteolytic elimination of misfolded or aggregated proteins, carried out by members of the AAA+ protein superfamily (ATPase associated with various cellular activities), such as Hsp100/Clp (heat shock protein-100/caseinolytic protease), Lon, and FtsH [2]. It has been estimated that the Clp and Lon families perform around 80% of cellular proteolysis in bacteria [3][4]. In addition, they control the proteolysis of regulatory proteins, such as key transcription factors that control the cell cycle and bacterial development or adaptation. These two opposite functions are regulated, in part, through the spatial and/or temporal use of adapter proteins, which participate in the recognition and delivery of specific substrate proteins to proteases [5][6].
The HSP100/Clp family of ATPases plays crucial roles in the folding, assembly, and degradation of proteins during normal growth and, mainly, under stress-inducing conditions [7][8]. This family is formed by several ATPase chaperones and the peptidase ClpP (caseinolytic protease proteolytic subunit). Chaperones are divided into two classes: Class I, whose members are ClpA (caseinolytic protease subunit (A), ClpB (caseinolytic protease subunit (B), and ClpC (caseinolytic protease subunit (C), which have two ATP-binding domains separated by a spacer region; and Class II, which includes ClpX (caseinolytic protease subunit X) and ClpY (caseinolytic protease subunit Y), that present only one domain of binding to ATP. Most of the chaperones bind to ClpP peptidase to form a proteolytic complex, with the exception of ClpY which only interacts with ClpQ, forming ClpQY peptidase, also known as HsIUV [9]. ClpQ is part of the Clp family, and like ClpP, is an ATP-dependent peptidase. However, it is one of the least-studied and its biological function and regulation are still not very clear. In addition, it has been shown to exhibit differences in the active site between Gram-positive and Gram-negative organisms [10].
The chaperone–ClpP complex is capable of degrading proteins in a specific manner where the chaperones can use ATP to promote protein folding changes and direct protein degradation by ClpP [11][12]. Although ClpP is part of this family, it does not have the same functions, since, unlike the rest, it is an ATP-dependent peptidase that, when associated with one of the chaperones, has serine protease activity [13]. Most bacteria contain the ClpXP protease, which makes ClpXP the most ubiquitous of the Clp proteases. On the other hand, ClpA and ClpC are orthologous; ClpA is usually found in Gram-negative bacteria, while ClpC is found in Gram-positive bacteria and cyanobacteria. ClpYQ exists together with ClpAP in most Gram-negative bacteria, and is also found in certain Gram-positive bacteria. Protein degradation dependent on these proteases has been studied in detail in the Gram-negative bacterium Escherichia coli (E. coli), whereas ClpCP has been characterized in the Gram-positive, spore-forming bacterium, Bacillus subtilis (B. subtilis) [9][14].
At the end of the 1990s, ClpP began to attract attention due to its potential as an antibacterial target, demonstrating that it participates in the bacterial virulence of the Gram-positive pathogens Staphylococcus aureus (S. aureus) and Listeria monocytogenes (L. monocytogenes). Since then, its use as a pharmaceutical target has been described in both Gram-positive and Gram-negative bacteria, being effective mostly in Gram-positive bacteria, where it proved to be effective in eliminating S. aureus, Enterococcus faecalis (E. faecalis), Streptococcus pneumoniae (S. pneumoniae), B. subtilis, and L. monocytogenes [9][15].
2. Clp Protease Families
To date, ATP-dependent proteases Lon, FtsH, and Clp have been characterized [1][2][16]. These complexes are responsible for maintaining a proper balance between protein synthesis and degradation at the cellular level, helping to maintain homeostasis. The peptidases of the Hsp100/Clp family are part of the quality control system of proteins both in normal growth and under stress conditions, playing an important role [17] (Figure 1 and Table 1).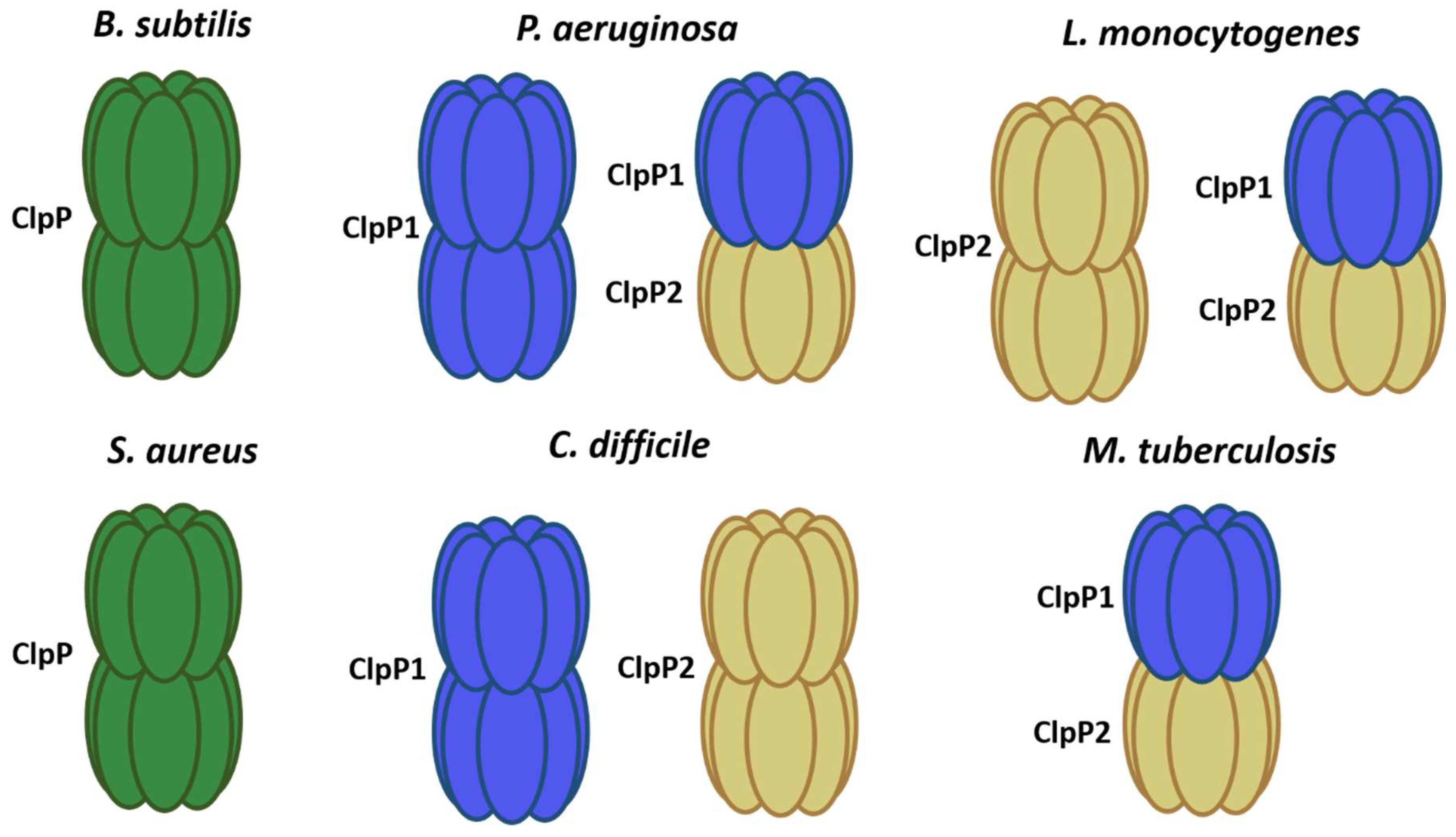
Figure 1. ClpP models in different bacteria. In green, it shows single ClpP peptidases forming 2 rings (B. subtilis and S. aureus). ClpP1 subunit in blue and ClpP2 in yellow.
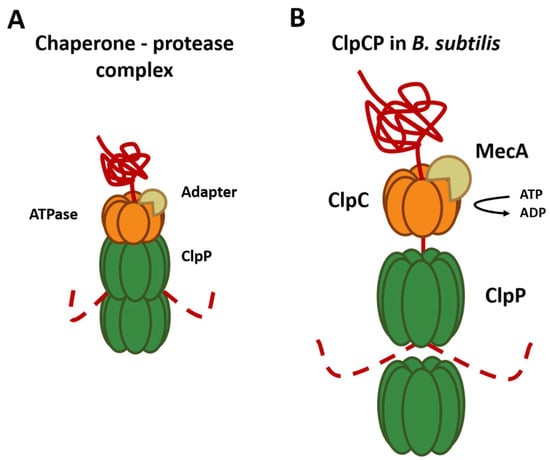
Figure 2. Composition and mechanism of the chaperone–peptidase complex. Substrates are recognized and unfolded by the hexameric chaperone (orange) using an adapter (brown). The unfolded substrate (red) is transferred into the proteolytic chamber of the ClpP (green), where proteolysis is carried out. (A) General composition of the chaperone protease complex. (B) Composition of the ClpCP chaperone–protease complex in B. subtilis.
Table 1. Different proteases and chaperones in Gram-positive bacteria.
Regulation of Complex
As mentioned above, chaperones can recognize the substrates that will be degraded by ClpP. The best-described substrate class comprises proteins tagged with the ssrA tag, a short peptide sequence C-terminally added to proteins by the tmRNA system to rescue stalled ribosomes. However, for this, the presence of specific substrate adapter proteins, such as YjbH, teicoplanin resistance factor designated A (TrfA), and McsB is required [32]. For example, ClpC requires adapter proteins for all its functions since it is only capable of forming a hexameric ring in the presence of the adapter protein MecA [33] (Figure 2B). These adapter proteins are also regulated through anti-adapter proteins or their phosphorylation of adapters mediated by different signals, for example, the anti-adapter protein ComS and the phosphorylation of the adapter proteins RssB and McsB. This series of regulations, in conjunction with the different Clp ATPases, allows an extensive regulation of the selection of the substrate that will be degraded by the ClpP peptidase. Other findings showed that the ClpP1P2 complex of M. tuberculosis requires the presence of an activating peptide; this peptide can be N-blocked dipeptide, usually Z-Leu-Leu (Benzyloxycarbonyl-L-Leucyl-L-Leucine), Z-Leu-Leu-H (Benzyloxycarbonyl-L-Leucyl-L-Leucinal), or a similar molecule, that binds near the active sites of the proteolytic particle and stabilizes the active conformation of the ClpP1P2 double-ring [34]. This functional conformation of the complex is also stabilized by the presence of the chaperone ClpX and protein substrate, acting synergistically with the activator peptide [35][36]. The requirement for activators seems to be a unique feature of Actinobacteria, as other species that contain two ClpP proteoforms, such as Listeria monocytogenes [37][38] and Chlamydia trachomatis [39], are functional in absence of activators. In the absence of proteolytic degradation via ClpP1P2, the levels of misfolded proteins can reach toxic levels, leading to cell death. Interestingly, E. coli ClpX rings can interact with M. tuberculosis ClpP1P2 complex and even promote substrate degradation more than ten-fold faster compared to the M. tuberculosis chaperones [36]. Another microorganism that possesses two isoforms of ClpP (ClpP1 and ClpP2) is C. difficile, but their functions are not yet known in detail. ClpP2 was only reported in hypervirulent strains, while ClpP1 has been reported in strains of ribotype 630 and hypervirulent strains, and it has been suggested that the ClpP isoforms act in an independent manner and possess different functions [40]. ClpP1 and ClpP2 have 74% and 63% identity, respectively, with ClpP from B. subtilis, an evolutionarily related organism, and sequence alignment showed that key regions including the catalytic triad are conserved. The evaluation of the proteolytic activity of each isoform was studied; however, only ClpP1 has proteolytic activity. On the other hand, it was revealed that both isoforms can form a complex with ClpX and degrade substrates labeled with the SsrA-tag, suggesting that the binding of the chaperone produces a change at the level of the ClpP2 peptidase that produces its activation [20].References
- Bhandari, V.; Wong, K.S.; Zhou, J.L.; Mabanglo, M.F.; Batey, R.A.; Houry, W.A. The Role of ClpP Protease in Bacterial Pathogenesis and Human Diseases. ACS Chem. Biol. 2018, 13, 1413–1425.
- Striebel, F.; Kress, W.; Weber-Ban, E. Controlled destruction: AAA ATPases in protein degradation from bacteria to eukaryotes. Curr. Opin. Struct. Biol. 2009, 19, 209–217.
- Maurizi, M.R.; Thompson, M.W.; Singh, S.K.; Kim, S. Endopeptidase Clp: ATP-dependent Clp protease from Escherichia coli. Methods Enzymol. Proteolytic Enzym. Serine Cysteine Pept. 1994, 244, 314–331.
- Goldberg, A.L.; Moerschell, R.P.; Hachung, C.; Maurizi, M.R. ATP-dependent protease La (Lon) from Escherichia coli. Methods Enzymol. Proteolytic Enzym. Serine Cysteine Pept. 1994, 244, 350–375.
- Frees, D.; Savijoki, K.; Varmanen, P.; Ingmer, H. Clp ATPases and ClpP proteolytic complexes regulate vital biological processes in low GC, Gram-positive bacteria. Mol. Microbiol. 2007, 63, 1285–1295.
- Kirstein, J.; Molière, N.; Dougan, D.A.; Turgay, K. Adapting the machine: Adaptor proteins for Hsp100/Clp and AAA proteases. Nat. Rev. Microbiol. 2009, 7, 589–599.
- Brötz-Oesterhelt, H.; Beyer, D.; Kroll, H.P.; Endermann, R.; Ladel, C.; Schroeder, W.; Hinzen, B.; Raddatz, S.; Paulsen, H.; Henninger, K.; et al. Dysregulation of bacterial proteolytic machinery by a new class of antibiotics. Nat. Med. 2005, 11, 1082–1087.
- Molière, N.; Hoßmann, J.; Schäfer, H.; Turgay, K. Role of Hsp100/Clp Protease Complexes in Controlling the Regulation of Motility in Bacillus subtilis. Front. Microbiol. 2016, 7, 315.
- Kress, W.; Mutschler, H.; Weber-Ban, E. Both ATPase Domains of ClpA Are Critical for Processing of Stable Protein Structures. J. Biol. Chem. 2009, 284, 31441–31452.
- Yu, Y.; Yan, F.; He, Y.; Qin, Y.; Chen, Y.; Chai, Y.; Guo, J.H. The ClpY-ClpQ protease regulates multicellular development in Bacillus subtilis. Microbiology 2018, 164, 848–862.
- Schirmer, E.C.; Glover, J.R.; Singer, M.A.; Lindquist, S. HSP100/Clp proteins: A common mechanism explains diverse functions. Trends Biochem. Sci. 1996, 21, 289–296.
- Lemos, J.A.; Burne, R.A. Regulation and Physiological Significance of ClpC and ClpP in Streptococcus mutans. J. Bacteriol. 2002, 184, 6357–6366.
- Gottesman, S.; Maurizi, M.R.; Wickner, S. Regulatory Subunits of Energy-Dependent Proteases. Cell 1997, 91, 435–438.
- Kruger, E. Clp-mediated proteolysis in Gram-positive bacteria is autoregulated by the stability of a repressor. EMBO J. 2001, 20, 852–863.
- Moreno-Cinos, C.; Goossens, K.; Salado, I.G.; Veken, P.V.; Winter, H.D.; Augustyns, K. ClpP Protease, a Promising Antimicrobial Target. Int. J. Mol. Sci. 2019, 20, 2232.
- Frees, D.; Thomsen, L.E.; Ingmer, H. Staphylococcus aureus ClpYQ plays a minor role in stress survival. Arch. Microbiol. 2005, 183, 286–291.
- Kruger, E.; Witt, E.; Ohlmeier, S.; Hanschke, R.; Hecker, M. The Clp Proteases of Bacillus subtilis Are Directly Involved in Degradation of Misfolded Proteins. J. Bacteriol. 2000, 182, 3259–3265.
- Akopian, T.; Kandror, O.; Raju, R.M.; Unnikrishnan, M.; Rubin, E.J.; Goldberg, A.L. The active ClpP protease from M. tuberculosis is a complex composed of a heptameric ClpP1 and a ClpP2 ring. EMBO J. 2012, 31, 1529–1541.
- Hall, B.M.; Breidenstein, E.B.; Fuente-Núñez, C.D.; Reffuveille, F.; Mawla, G.D.; Hancock, R.E.; Baker, T.A. Two Isoforms of Clp Peptidase in Pseudomonas aeruginosa Control Distinct Aspects of Cellular Physiology. J. Bacteriol. 2017, 199, e00568-16.
- Lavey, N.P.; Shadid, T.; Ballard, J.D.; Duerfeldt, A.S. Clostridium difficile ClpP Homologues are Capable of Uncoupled Activity and Exhibit Different Levels of Susceptibility to Acyldepsipeptide Modulation. ACS Infect. Dis. 2019, 5, 79–89.
- Olivares, A.O.; Baker, T.A.; Sauer, R.T. Mechanical Protein Unfolding and Degradation. Annu. Rev. Physiol. 2018, 80, 413–429.
- Gerth, U.; Kock, H.; Kusters, I.; Michalik, S.; Switzer, R.L.; Hecker, M. Clp-Dependent Proteolysis Down-Regulates Central Metabolic Pathways in Glucose-Starved Bacillus subtilis. J. Bacteriol. 2007, 190, 321–331.
- Frees, D.; Gerth, U.; Ingmer, H. Clp chaperones and proteases are central in stress survival, virulence and antibiotic resistance of Staphylococcus aureus. Int. J. Med. Microbiol. 2014, 304, 142–149.
- Ujiie, H.; Matsutani, T.; Tomatsu, H.; Fujihara, A.; Ushida, C.; Miwa, Y.; Fujita, Y.; Himeno, H.; Muto, A. Trans-Translation is Involved in the CcpA-Dependent Tagging and Degradation of TreP in Bacillus subtilis. J. Biochem. 2008, 145, 59–66.
- Sauer, R.T.; Baker, T.A. AAA Proteases: ATP-Fueled Machines of Protein Destruction. Annu. Rev. Biochem. 2011, 80, 587–612.
- Schlothauer, T.; Mogk, A.; Dougan, D.A.; Bukau, B.; Turgay, K. MecA, an adaptor protein necessary for ClpC chaperone activity. Proc. Natl. Acad. Sci. USA 2003, 100, 2306–2311.
- Turgay, K.; Hahn, J.; Burghoorn, J.; Dubnau, D. Competence in Bacillus subtilis is controlled by regulated proteolysis of a transcription factor. EMBO J. 1998, 17, 6730–6738.
- Pan, Q.; Garsin, D.A.; Losick, R. Self-Reinforcing Activation of a Cell-Specific Transcription Factor by Proteolysis of an Anti-σ Factor in B. subtilis. Mol. Cell 2001, 8, 873–883.
- Nakano, S.; Zheng, G.; Nakano, M.M.; Zuber, P. Multiple Pathways of Spx (YjbD) Proteolysis in Bacillus subtilis. J. Bacteriol. 2002, 184, 3664–3670.
- Miethke, M.; Hecker, M.; Gerth, U. Involvement of Bacillus subtilis ClpE in CtsR Degradation and Protein Quality Control. J. Bacteriol. 2006, 188, 4610–4619.
- Benaroudj, N.; Raynal, B.; Miot, M.; Ortiz-Lombardia, M. Assembly and proteolytic processing of mycobacterial ClpP1 and ClpP2. BMC Biochem. 2011, 12, 61.
- Donegan, N.P.; Marvin, J.S.; Cheung, A.L. Role of adaptor TrfA and ClpPC in controlling levels of SsrA-tagged proteins and antitoxins in Staphylococcus aureus. J. Bacteriol. 2014, 196, 4140–4151.
- Kirstein, J.; Schlothauer, T.; Dougan, D.A.; Lilie, H.; Tischendorf, G.; Mogk, A.; Bukau, B.; Turgay, K. Adaptor protein controlled oligomerization activates the AAA protein ClpC. EMBO J. 2006, 25, 1481–1491.
- Famulla, K.; Sass, P.; Malik, I.; Akopian, T.; Kandror, O.; Alber, M.; Hinzen, B.; Ruebsamen-Schaeff, H.; Kalscheuer, R.; Goldberg, A.L.; et al. Acyldepsipeptide antibiotics kill mycobacteria by preventing the physiological functions of the ClpP1P2 protease. Mol. Microbiol. 2016, 101, 194–209.
- Leodolter, J.; Warweg, J.; Weber-Ban, E. The Mycobacterium tuberculosis ClpP1P2 Protease Interacts Asymmetrically with Its ATPase Partners ClpX and ClpC1. PLoS ONE 2015, 10, e0125345.
- Schmitz, K.R.; Sauer, R.T. Substrate delivery by the AAA ClpX and ClpC1 unfoldases activates the mycobacterial ClpP1P2 peptidase. Mol. Microbiol. 2014, 93, 617–628.
- Zeiler, E.; List, A.; Alte, F.; Gersch, M.; Wachtel, R.; Poreba, M.; Drag, M.; Groll, M.; Sieber, S.A. Structural and functional insights into caseinolytic proteases reveal an unprecedented regulation principle of their catalytic triad. Proc. Natl. Acad. Sci. USA 2013, 110, 11302–11307.
- Dahmen, M.; Vielberg, M.; Groll, M.; Sieber, S.A. Structure and Mechanism of the Caseinolytic Protease ClpP1/2 Heterocomplex from Listeria monocytogenes. Angew. Chem. Int. Ed. 2015, 54, 3598–3602.
- Pan, S.; Malik, I.T.; Thomy, D.; Henrichfreise, B.; Sass, P. The functional ClpXP protease of Chlamydia trachomatis requires distinct clpP genes from separate genetic loci. Sci. Rep. 2019, 9, 14129.
- Sekulovic, O.; Fortier, L. Global Transcriptional Response of Clostridium difficile Carrying the ϕCD38-2 Prophage. Appl. Environ. Microbiol. 2014, 81, 1364–1374.
More
