Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Elena Pedrini.
Osteopetrosis (from the Greek “osteo”: bone; “petrosis”: stone) is a clinically and genetically heterogeneous group of rare diseases of the skeleton, sharing the same main characteristic of an abnormally increased bone density. Dense bones in radiological studies are considered the hallmark of these diseases, and the reason for the common term used: “Marble bone disease”.
- radiography
- magnetic resonance imaging
- multidetector computed tomography
- osteopetrosis
1. Introduction
Osteopetrosis refers to a clinically and genetically heterogeneous group of rare, inheritable disorders of the skeleton, characterized by an abnormal increase in bone density on radiographs. For this reason, osteopetrosis is also called ‘marble bone disease’ [1,2][1][2]. This condition results from an osteoclast dysfunction or abnormal osteoclast differentiation, leading to a defect in bony resorption. The bone mass appears to be increased and abnormally dense with characteristic bone brittleness.
Osteopetrosis was originally described by the radiologist Dr. Albers-Schonberg in Germany in 1904, even though the term derives from Greek (“osteo”: bone; “petrosis”: stone) [3].
The different osteopetrosis groups also arise from different inheritable conditions, all characterized by a defect in bone resorption, abnormal bone growth and remodelling, osteosclerosis, and higher bony brittleness [4]. The typical radiographic finding is an abnormally increased bone density, resulting in a frequent ‘bone within a bone’ appearance [5,6][5][6]. The atypical osteoclast activity also alters the bony shape and structure, modifying its capacity to remodel during growth. In addition, the medullary cavity becomes filled with endochondral new bone, which leaves little space for hematopoietic cells, causing higher bony brittleness [4].
Four main types of disease have been identified based on different models of inheritance and severity [3,4][3][4]:
The infantile or ‘malignant’ form: it is an autosomal recessive osteopetrosis (ARO), typically diagnosed within the first year of birth and characterized by a high severity that could lead to mortality in early childhood [6,7][6][7]. This form includes the so-called X-linked variant of osteopetrosis, an extremely rare and clinically severe disease, with a recessive transmission such as the other ‘malignant’ form. It can be noted that only a few patients with X-linked type osteopetrosis are reported in the literature.
The ‘intermediate’ autosomal osteopetrosis (IAO): this form is due to an autosomal recessive transmission, which usually becomes clinically significant during the first decade of life. The typical signs are pathologic fractures and progressive cranial nerve compression neuropathies. This form is milder than the previous condition and the affected patients typically reach adulthood.
The autosomal dominant osteopetrosis (ADO), type I: patients are often asymptomatic and, in general, the fracture risk is not increased. The typical sign appears as an isolated osteosclerotic thickening of the cranial vault.
The ADO, type II (Albert-Schönberg disease): even for this form, patients may often be asymptomatic for a long time, but it will eventually present in adulthood with anaemia, pathologic fracture, bone pain, cranial nerve compression, or early arthritis [8].
Mutations involving at least eight genes have been identified as responsible for osteopetrosis pathogenesis in humans [1,9][1][9]. Six of these eight genes have been identified in the malignant form (TCIRG1, OSTM1, PLEKHM1, SNX10, TNFSF11, TNFRSF11A), while the mutation of the CLCN7 gene (which codes for the chloride channel 7 protein) has been identified both in ARO and ADO (types I and II). Instead, the IAO results from a loss of function mutation in CAII, the gene responsible for the carbonic anhydrase II protein [9]. Because mutations in the genes described so far only account for approximately 70% of cases, it is highly likely that additional genes involved in the pathogenesis of osteopetrosis will be discovered.
Radiological studies are fundamental for the clinical diagnosis of osteopetrosis. They are able to assess the severity of the disease, usually associated with different known genetic mutations. If typical radiographic signs for osteopetrosis are missing, raised blood concentrations of the creatine kinase BB isoenzyme and tartrate resistant acid phosphatase (TRAP) can be helpful for diagnosis. In addition, genetic testing should be performed to evaluate the presence of a gene mutation, because the distinction between different subtypes is important given the different response to treatments, prognosis, and recurrence risks.
2. Imaging of Osteopetrosis
The diagnosis of osteopetrosis is clinical, based on history and physical examination, and radiological as well. Imaging plays a useful role in detecting the typical radiological manifestations and identifying sites of skeletal involvement [13][10]. Indeed, imaging investigations are central to establishing a clinical diagnosis of osteopetrosis [14][11]. In severe forms, radiological examinations can provide rapid confirmation of the suspected diagnosis. On the contrary, in milder forms, imaging findings may be the first or even the only signs of the disease [15][12]. Various imaging techniques may be helpful for the assessment of the different features of the disease in the skeleton, and also to evaluate complications. Conventional radiography alone can show the main well-recognized patterns of osteopetrosis, and above all the abnormal increased bone density. Computed Tomography (CT) can detect all the patterns of osteopetrosis as well, offering a higher level of detail and higher spatial resolution than conventional radiography. CT could be helpful in better detecting possibly associated fractures or inflammatory diseases of the bone. Dual-energy X-ray absorptiometry (DXA) is a radiological tool able to measure bone mineral density (BMD) in a quantitative manner. DXA shows the elevation of BMD in patients affected by osteopetrosis due to the pathologically increased bone density [16][13]. Even for the mildest forms of osteopetrosis, bone density is markedly elevated on DEXA evaluation, with Z-scores usually of +5 standard deviations or greater [15,17][12][14]. DXA is particularly useful in the follow up of the disease, as it has the ability to detect and quantify the progression and/or response to treatments [18,19][15][16]. Unfortunately, despite the utility of DXA in the general population, in which low bone mineral density is a well-known risk factor for fracture, this tool is not able to accurately predict the risk for fractures in patients with osteopetrosis [20][17]. Nuclear medicine examinations can be used to assess osteopetrosis. Particularly, 99mTc-sulfur colloid scintigraphy can be performed to detect the bone marrow distribution of the disease [18][15]. Moreover, 99mTc-MDP and other bone scans can be used to show uptake at multiple fracture sites. SPECT/CT help with accurately localizing, confirming, or excluding any abnormal sites of increased metabolic activity [18][15]. Magnetic resonance imaging (MRI) is useful in more severe cases to evaluate the amount of remaining bone marrow space. In addition, an MRI of the brain and orbits could be employed to assess the narrowing of central nerve channels, resulting in nerve compression and neuropathic changes.2.1. Pathophysiological Explanations of Skeletal Imaging Findings
The imaging features in all forms of osteopetrosis are explained by the disordered osteoclast function, which is the central biological defect. The pathophysiology of the disease is characterized by an imbalance between osteoblastic bone addition and osteoclastic bone removal, leading to an unopposed increase in bone mass and density [15][12]. This results in an increase in bone density (considered the hallmark of the disease), an increase in cortical bone volume (hyperostosis), and in severe cases, leads to a loss of medullary cavities, defective bone modelling (undermodelling or undertubulation), bone fragility with an increased risk of fractures, and bone deformities [15][12]. Osteoclasts also play a key role in the shaping of bones during growth. Normal long bones of healthy subjects are wider at their epiphyseal, joint forming ends, and taper towards their centre to optimise the load-bearing-to-weight ratio [15][12]. The conversion of a wider metaphysis to a narrower diaphysis (metaphyseal remodelling) is critically dependent on the normal function of osteoclasts; thus, in osteopetrosis, the long bones are ‘undermodelled’, presenting with an elongated transition from wide metaphysis to narrow diaphysis (especially in the distal femur). The trabecular formation of the secondary spongiosa and the osteoclastic resorption on the endosteal surface are critical for the creation of bone marrow spaces; indeed, this space is diminished or absent in severe osteopetrosis forms, and it is associated with consequent bone marrow failure [15][12].2.2. Pattern of Imaging Features
The most recognized and frequent radiological signs of osteopetrosis can be summarized in four classic imaging features that may appear in the radiological evaluation of patients affected by the disease: (1) Diffuse increased bone density, sclerosis involving the skull, spine, pelvis, and appendicular bones: This sign is the most known and recognized imaging feature of osteopetrosis, and the reason for the name ‘marble bone disease’ of this condition. Diffuse bone sclerosis can be found in all subtypes of osteopetrosis (ADO, ARO, IAO), even if with slightly different patterns and skeletal locations; particularly in the ARO type, the bone sclerosis can be so marked to assume the so-called ‘marble bone’ appearance (Figure 1).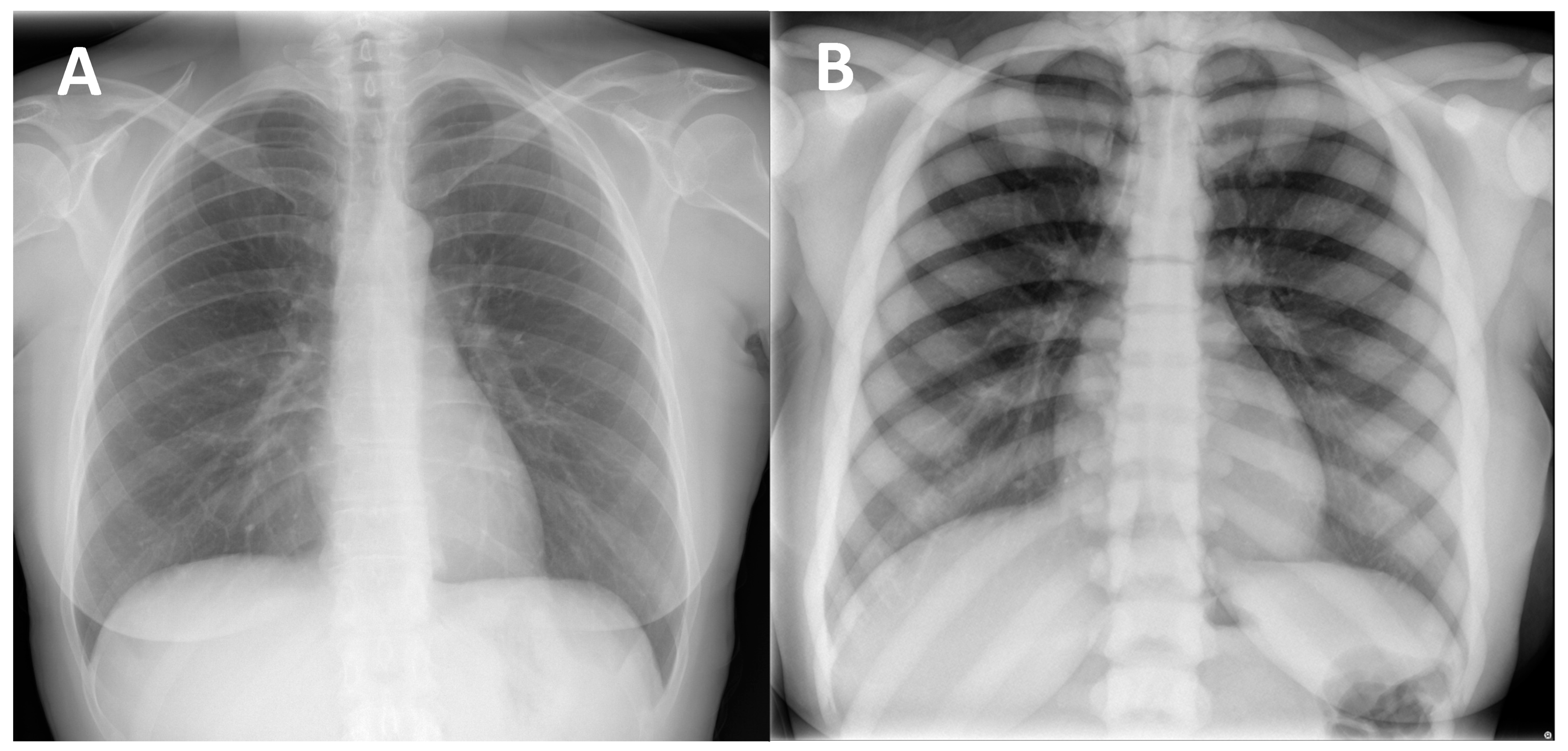
Figure 1. (A) conventional radiography of the chest of a young healthy female (shown as comparison). (B) conventional radiography of the chest in a 29-year-old female diagnosed with osteopetrosis (clinical and radiological diagnosis in absence of a pathogenic variant in osteopetrosis-related genes). All the skeletal segments included are affected by diffuse and markedly increased bone density (spine, ribs, clavicle, scapulae, humerus): ‘Marble bone’ appearance.
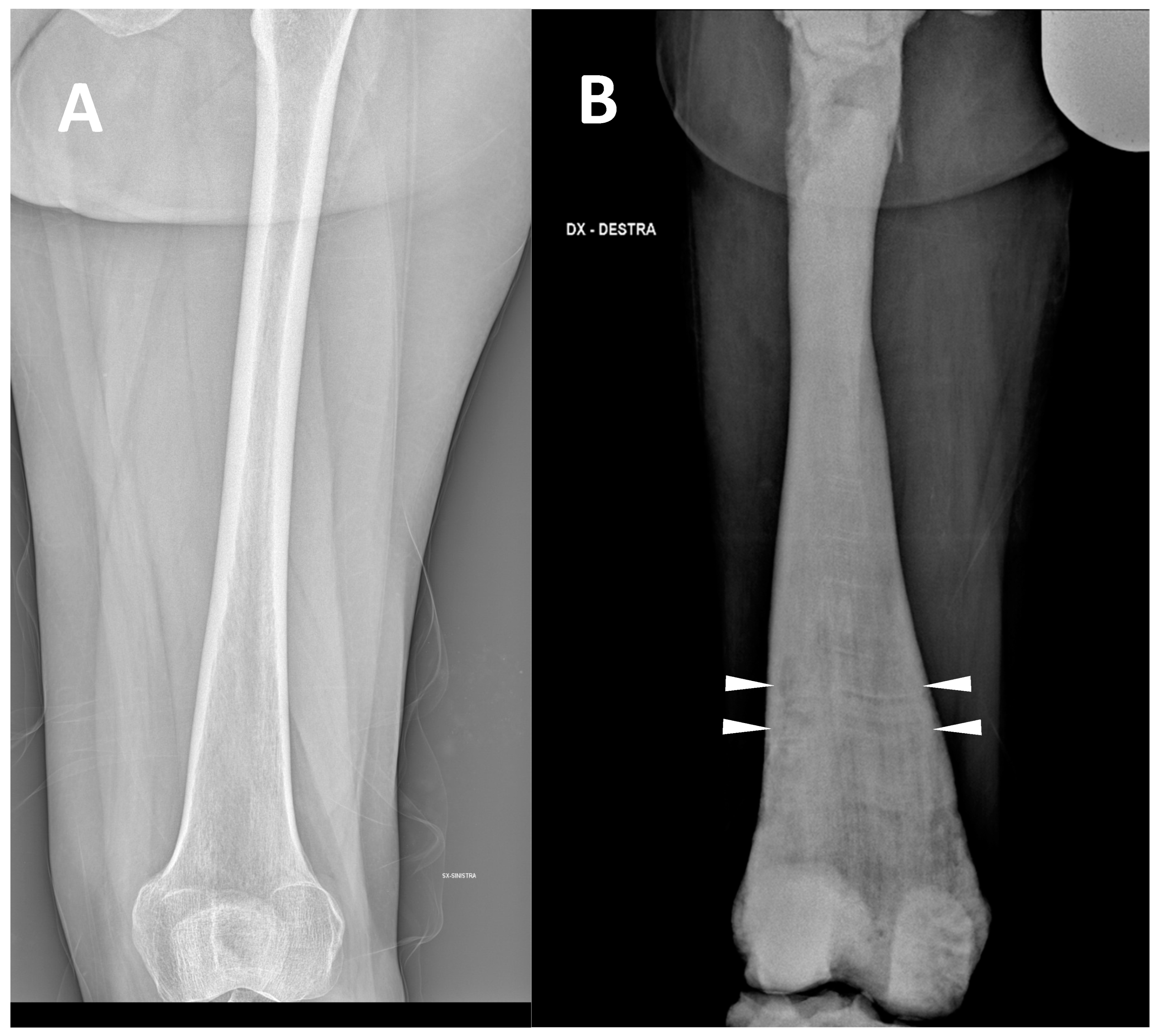
Figure 2. (A) conventional radiography of the thigh of a young healthy female (shown as comparison). (B) conventional radiography of the right thigh in a 35-year-old male affected by osteopetrosis (ARO type). Metaphyseal enlargement with the so-called ‘Erlenmeyer flask deformity’ of the distal femur, as well as a diffuse increase in bone radiolucency (increased bone density) are detectable. Alternating lucent and sclerotic metaphyseal banding can also be noted.
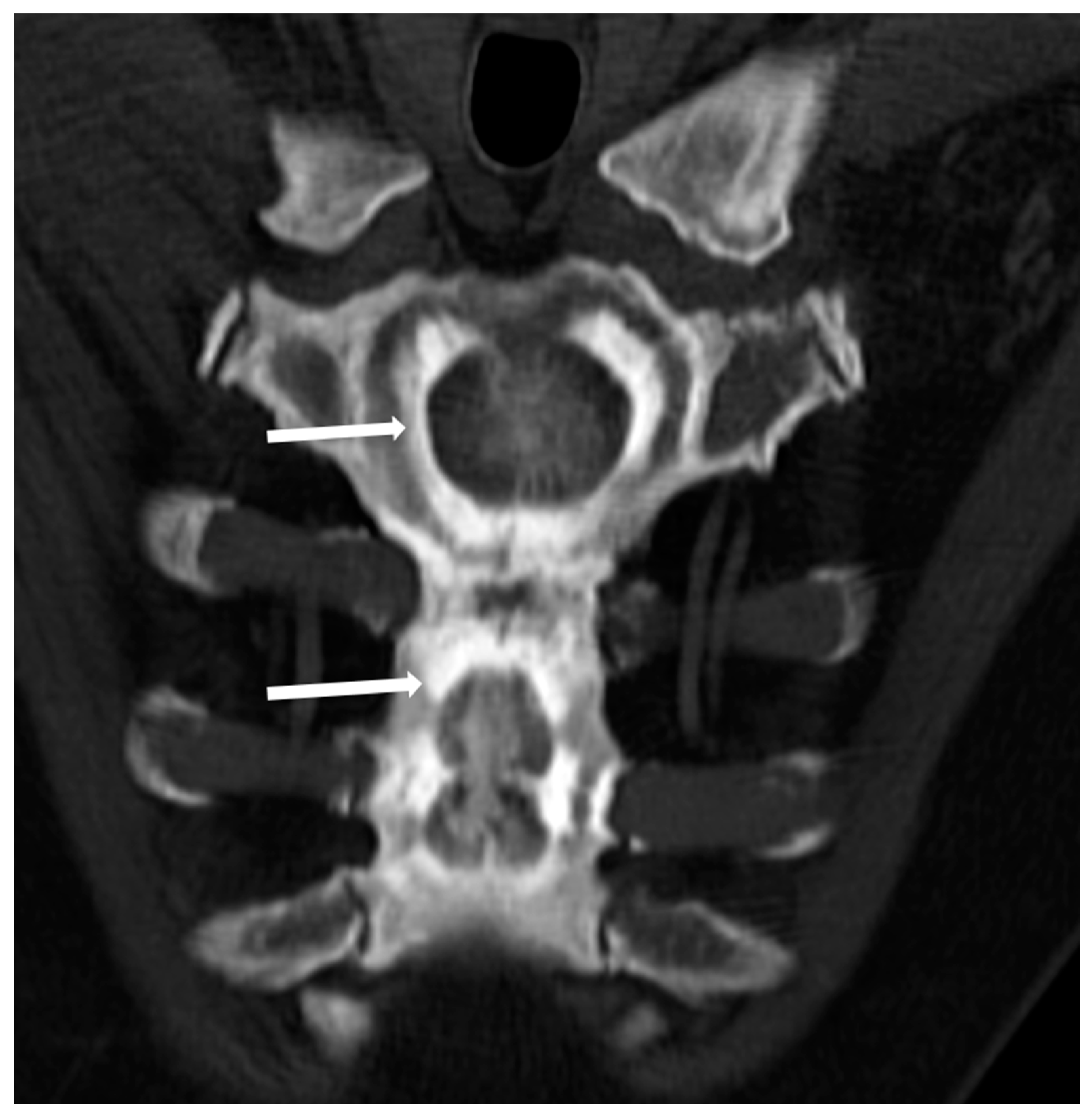
Figure 3. Computed Tomography (CT, coronal-oblique reconstruction) of the sternum in a 48-year-old male affected by osteopetrosis (ADO type 2). The so-called ‘bone in bone’ appearance is detectable with a well-defined increased density within the skeletal segment involved (arrows).
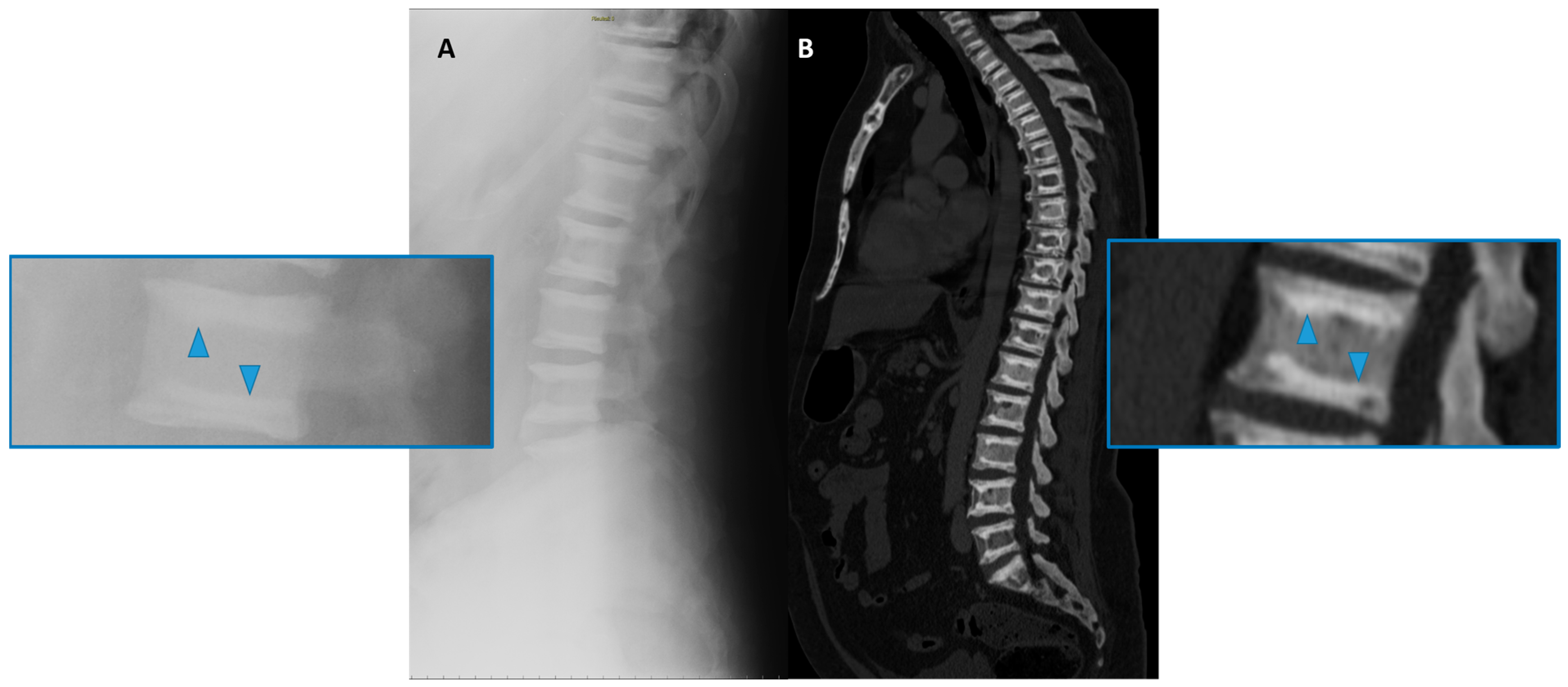
Figure 4. Conventional radiography (lateral projection, (A)) and Computed Tomography (CT Sagittal reconstruction, (B)) of the spine of a 50-year-old male affected by osteopetrosis (ADO type 2). Well-defined areas of increased bone density are detectable on the vertebral endplates (arrowhead), configuring the so-called ‘sandwich vertebrae’ appearance.
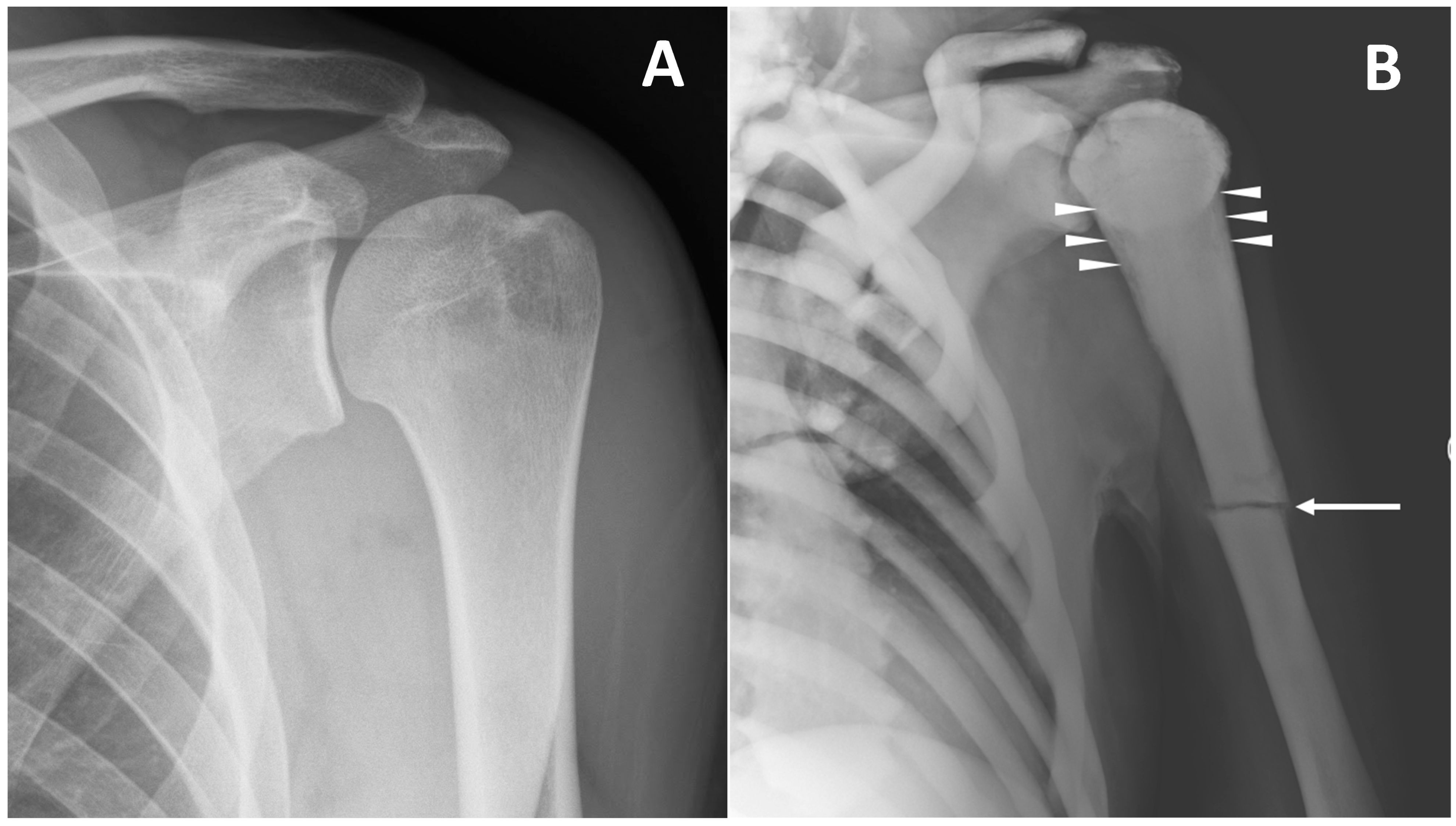
Figure 5. (A) conventional radiography of the left shoulder in a young healthy male (shown as comparison). (B) conventional radiography of a 45-year-old male with ARO type of osteopetrosis presenting with atraumatic pathological fracture of the diaphysis of the left humerus (arrow). A mild alteration of proximal metaphysis shape (arrowheads), and a diffuse increase in bone density of all the skeletal sites included can also be detected.
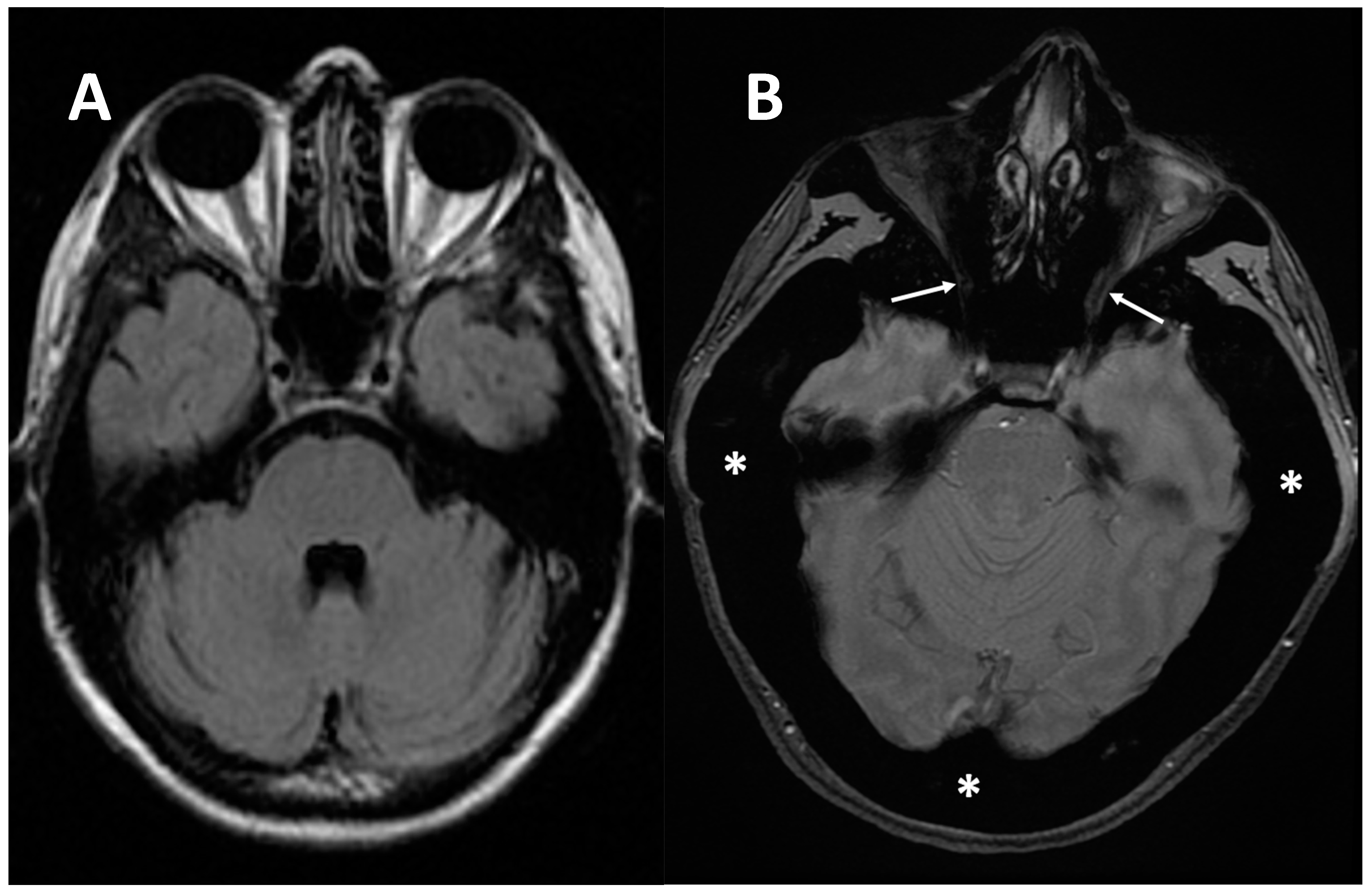
Figure 6. (A) axial MRI of a healthy young female (shown as comparison). (B) axial MRI (T2w*) of a 38-year-old female with a clinical-radiological diagnosis of osteopetrosis (ARO type); narrowing of both optic foramens can be noted (arrows) caused by a diffuse bone thickening. Diffuse bone thickening of cranial bones is associated with a marked ipointense signal intensity of all the skeletal structures related to the diffuse bone sclerosis (asterisks).
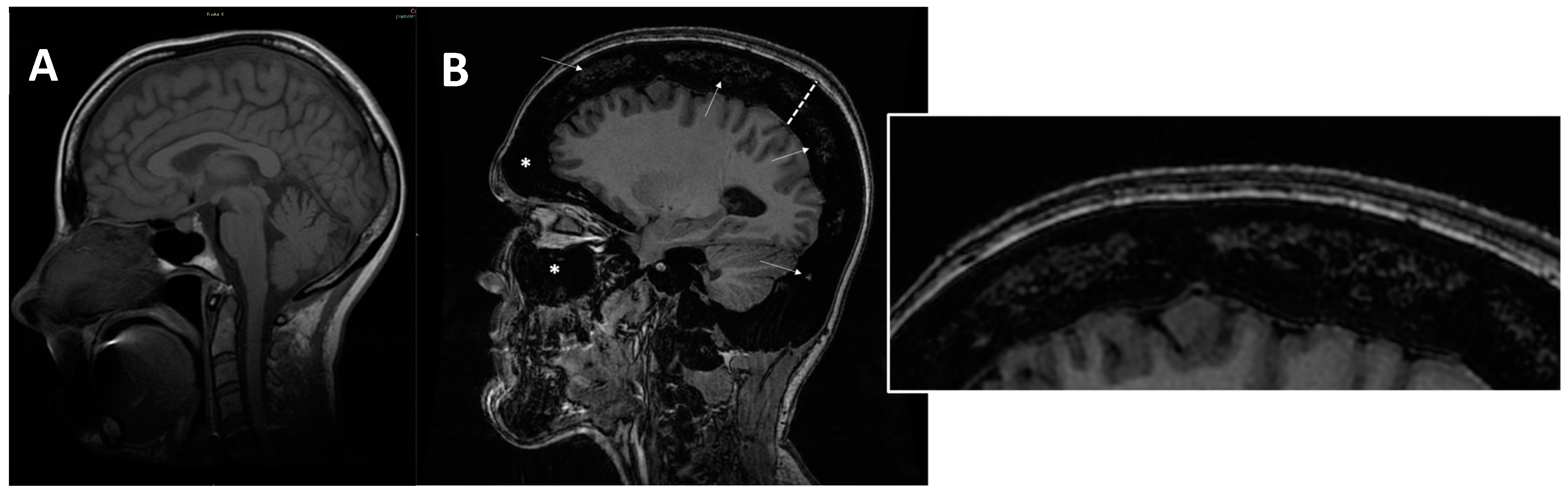
Figure 7. (A) axial MRI of a healthy young female (shown as comparison). (B) sagittal MRI (proton density sequence) in ARO-type osteopetrosis patient suffering from anaemia (Hb = 7g/dL) shows markedly reduced bone marrow space (arrows and enlargement on the right); diffuse calvarias bone thickening (dotted line), sclerosis (signal hypointensity), and obliteration of maxillary and frontal paranasal sinus (asterisks) can be noted.
References
- Stark, Z.; Savarirayan, R. Osteopetrosis. Orphanet. J. Rare Dis. 2009, 4, 5.
- Bailey, J.R.; Tapscott, D.C. Osteopetrosis. In StatPearls. Treasure Island; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Albers-Schönberg, H.E. Rontgenbilder einer seltenen Knockenerkrankung. Munch Med. Wochenschr. 1904, 5, 365–368.
- Tolar, J.; Teitelbaum, S.L.; Orchard, P.J. Osteopetrosis. N. Engl. J. Med. 2004, 351, 2839–2849.
- Shapiro, F. Osteopetrosis: Current clinical considerations. Clin. Orthop. 1993, 7, 34–44.
- Loría-Cortés, R.; Quesada-Calvo, E.; Cordero-Chaverri, C. Osteopetrosis in children: A report of 26 cases. J. Pediatr. 1977, 91, 43–47.
- Beighton, P.; Horan, F.; Hamersma, H. A review of the osteopetroses. Postgrad. Med. J. 1977, 53, 507–516.
- de Baat, P.; Heijboer, M.P.; de Baat, C. Osteopetrosis. Classification, etiology, treatment options and implications for oral health. Ned. Tijdschr. Tandheelkd. 2005, 112, 497–503.
- Coudert, A.E.; de Vernejoul, M.C.; Muraca, M.; Del Fattore, A. Osteopetrosis and its relevance for the discovery of new functions associated with the skeleton. Int. J. Endocrinol. 2015, 2015, 372156.
- Stoker, D.J. Osteopetrosis. Semin. Musculoskelet Radiol. 2002, 6, 299–305.
- Wu, C.C.; Econs, M.J.; DiMeglio, L.A.; Insogna, K.L.; Levine, M.A.; Orchard, P.J. Diagnosis and Management of Osteopetrosis: Consensus Guidelines from the Osteopetrosis Working Group. J. Clin. Endocrinol. Metab. 2017, 102, 3111–3123.
- Calder, A.D.; Arulkumaran, S.; D’Arco, F. Imaging in osteopetrosis. Bone 2022, 116560.
- Kaste, S.C.; Kasow, K.A.; Horwitz, E.M. Quantitative bone mineral density assessment in malignant infantile osteopetrosis. Pediatr. Blood Cancer. 2007, 48, 181–185.
- Ladd, L.M.; Imel, E.A.; Niziolek, P.J.; Liu, Z.; Warden, S.J.; Liang, Y.; Econs, M.J. Radiographic imaging, densitometry and disease severity in Autosomal dominant osteopetrosis type 2. Skelet. Radiol. 2021, 50, 903–913.
- Sit, C.; Agrawal, K.; Fogelman, I.; Gnanasegaran, G. Osteopetrosis: Radiological & radionuclide imaging. Indian J. Nucl. Med. 2015, 30, 147544.
- Alam, I.; Gerard-O’Riley, R.L.; Acton, D.; Hardman, S.L.; Murphy, M.; Alvarez, M.B.; Blosser, R.J.; Sinn, A.; Srour, E.F.; Kacena, M.A.; et al. Bone marrow transplantation as a therapy for autosomal dominant osteopetrosis type 2 in mice. FASEB J. 2022, 36, e22471.
- Arruda, M.; Coelho, M.C.; Moraes, A.B.; de Paula Paranhos-Neto, F.; Madeira, M.; Farias, M.L.; Vieira Neto, L. Bone Mineral Density and Microarchitecture in Patients with Autosomal Dominant Osteopetrosis: A Report of Two Cases. J. Bone Min. Res. 2016, 31, 657–662.
- Faden, M.A.; Krakow, D.; Ezgu, F.; Rimoin, D.L.; Lachman, R.S. The Erlenmeyer flask bone deformity in the skeletal dysplasias. Am. J. Med. Genet. A 2009, 149, 1334–1345.
- Adusumilli, G.; Kaggie, J.D.; D’Amore, S.; Cox, T.M.; Deegan, P.; MacKay, J.W.; McDonald, S. GAUCHERITE Consortium. Improving the quantitative classification of Erlenmeyer flask deformities. Skeletal Radiol. 2021, 50, 361–369.
- Dähnert, W.F. Radiology Review Manual; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011; ISBN 1609139437.
- Williams, H.; Davies, A.; Chapman, S. Bone within a bone. Clin. Radiol. 2004, 59, 132–144.
- Kirkland, J.D.; O’Brien, W.T. Osteopetrosis—Classic Imaging Findings in the Spine. J. Clin. Diagn. Res. 2015, 9, TJ01.
- Appelman-Dijkstra, N.M.; Navas Cañete, A.; Soonawala, D. The rugger-jersey spine. Kidney Int. 2016, 90, 454.
More
