You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Kin Wah Terence Lee.
Hepatocellular carcinoma (HCC) is a major cause of cancer death worldwide due to its high rates of tumor recurrence and metastasis. Aberrant Wnt/β-catenin signaling has been shown to play a significant role in HCC development, progression and clinical impact on tumor behavior.
- cancer metabolism
- drug resistance
- metabolic reprogramming
- hepatocellular carcinoma
- Wnt/β-catenin
1. Mutation and Expression Status of the Wnt/β-Catenin Pathway and Its Clinical Significance
Wnt/β-catenin signaling is crucial in contributing to HCC pathogenesis, where genetic mutations and epigenetic alterations are primarily revealed [10][1]. Activation of the Wnt/β-catenin signaling pathway was discovered in 20–35% of HCC cases, among which most are resulted by gene mutations of the key genes, including CTNNB1, AXIN, and APC [16,17,18][2][3][4]. In this case, CTNNB1 is the gene that specifically encodes β-catenin. Mutation of β-catenin is positively related to HCC progression due to its oncogenic role [19][5]. To date, mutations at the serine/threonine sites of exon 3 of the β-catenin gene are mostly found to be involved in the phosphorylation and ubiquitination of β-catenin, thus enhancing its nuclear translocation in approximately 20% of HCC cases [20,21][6][7]. In addition, conventional and missense mutations have also been reported in other codons of β-catenin [22][8]. Previous reports showed that conventional mutations at codons 33, 37, 41, and 45 are discovered in over 12% of HCC patients, where missense mutations are observed at codons 32, 34, and 35 [22][8], which indicates the capability of mutated β-catenin proteins to evade degradation and enter the nucleus [20,23][6][9]. It is also noted that tumor cells with aberrant Wnt/β-catenin activation due to the mutation of β-catenin that tend to grow and spread more quickly in HCC [19][5].
Apart from β-catenin, deregulation of the Wnt/β-catenin signaling pathway is also caused by mutations in protein degradation complexes [24][10]. These mutations cause dysfunction of the destruction complex and accumulation of β-catenin in the nucleus in approximately 40–70% of HCC cases [10][1]. One example is the amino acid substitution in armadillo repeats domain 5/6 of β-catenin in human HCC cases [25][11]. This results in a reduction of APC binding to the degradation complex, which activates the Wnt/β-catenin signaling pathway and enhances targeted gene transcription [25][11]. It has been reported that a small amount of β-catenin accumulated in the nucleus is sufficient to activate Wnt target genes, suggesting the crucial role of β-catenin in HCC progression [26,27][12][13]. However, several studies have shown that the mutation of β-catenin alone is insufficient for promoting HCC in mice, which is different in comparison with humans [27[13][14],28], as the tumorigenic potential could be augmented when combined with other oncogenic pathways, such as H-RAS, MET, AKT, or chemicals such as diethylnitrosamine (DEN) [10,29][1][15].
In addition, high levels of E-cadherin have been reported to be correlated with the accumulation of β-catenin in both the cytosol and nucleus, that drives the transcription of Wnt target genes [9][16]. C-Myc and cyclin D, as key Wnt-target genes, not only perform their roles as proto-oncogenes for tumor formation but also regulate liver cancer stem cell (CSC) properties by mediating various signaling pathways involved in cellular differentiation and survival [19,20][5][6]. As previously mentioned, HBV and HCV are the causes of HCC, in which they lead to genetic mutations in genes involved in Wnt/β-catenin signaling [9,30][16][17]. It is common to find CTNNB1 mutations in HCV-related HCC rather than HBV-related HCC or nonviral HCC [24][10]. However, mutation of Axin1 is more often found in HBV-related HCC tumors [24][10]. Interestingly, apart from the mutations of the canonical pathway, Zucman-Rossi et al. suggested that Axin1 mutation also plays a role in exerting oncogenic effects manifested by overexpressing glutamine synthase (GS), leading to β-catenin activation that correlates to the non-canonical pathway [31][18].
2. Regulation of Wnt/β-Catenin Pathway in HCC
2.1. Epigenetic Regulation of Wnt/β-Catenin
Several epigenetic dysregulations contribute to Wnt/β-catenin activation in HCC. DNA methylation is crucial in maintaining CSC properties, in which its inhibition can influence the fate of cells and gene expressions [32][19]. For instance, DNA methyltransferase (DNMT) plays a role in catalyzing the transition between a methyl group and DNA, mediating BEX1 expression in HCC [33][20]. A decrease in DNMT1 results in BEX1 hypomethylation that further enhances the transcription of β-catenin, which causes the activation of the Wnt/β-catenin signaling pathway [33][20]. Moreover, secreted frizzled-related proteins (SFRPs) negatively regulate Wnt/β-catenin signaling via DNA methylation, representing a leading cause of activating β-catenin activity in HCC [34][21]. Another study also consistently showed that downregulation of the SFRP family is correlated with Wnt/β-catenin signaling activation, in which SFRP1 and SFRP5 are also found to enhance the progression of HCC [35,36][22][23]. Similarly, downregulating Wnt inhibitory factor 1 (WIF1) or Dickkopf-related protein 3 (DKK3) has been proven to result in common consequences for SFRPs [37][24]. In addition, SOX17 is reported to take part in the aberrant activation of Wnt/β-catenin signaling due to promoter methylation [38][25]. Silencing of SOX17 could enhance Wnt activity due to the failure in interacting with TCF/LEF, which hinders Wnt-target gene transcription [39][26]. Apart from the genomic instability caused by DNA hypomethylation, another study showed the involvement of potassium channels in epigenetic regulation of the Wnt/β-catenin pathway [40][27]. Fan et al. revealed that a decrease in KCNQ1 (potassium voltage-gated channel subfamily Q member 1) causes an increment in Wnt/β-catenin activity via DNA hypermethylation [40][27].
Furthermore, several alterations through histone modification have been reported in HCC. Enhancer of zeste homologous 2 (EZH2) is a histone methyltransferase that plays a role in catalyzing methylation of histone H3 to achieve repression of Wnt antagonists, promoting Wnt/β-catenin signaling and hepatocarcinogenesis [41,42][28][29]. Histone deacetylases (HDAC) have been revealed to interact with EZH2 through its enzymatic role [43][30]. Specifically, for HDAC8, its upregulation due to the chromatin modifications is coexpressed with the lipogenic transcription factor SREBP1 in HCC mouse models, causing cell cycle arrest and β-catenin activation, which drives NAFLD-induced hepatocarcinogenesis [43][30]. Moreover, HDAC8 can also bind to pyruvate kinase M2 (PKM2) and subsequently deacetylate the residue K62, prompting the nuclear translocation of PKM2 and the binding of β-catenin that results in Wnt target gene transcription [44][31]. Similarly, EZH2 overexpression elevated the levels of the oncogene H3K27me3, which silenced Wnt inhibitors, leading to induced cell proliferation with activated β-catenin activity [41][28].
2.2. Non-Coding RNAs in Regulation of Wnt/β-Catenin
It has been suggested that microRNAs (miRNAs) and long noncoding RNAs (lncRNAs) are critical regulators associated with various tumors, in which they are negatively regulated [45][32]. Dysregulation of miRNAs and lncRNAs could lead to tumorigenesis in HCC. LncRNA-miRNA binding yields a complete endogenous RNA (ceRNA) that can avoid messenger RNA (mRNA) recognition and further silencing effects, known as the “sponge effect” [45][32]. Mounting evidence suggests that miRNA sponges are involved in Wnt/β-catenin signaling and are associated with HCC progression (Table 1). For example, LINC00355 and LINC01278 are negative regulators of miR-217-5p and miR-1258, respectively [46,47][33][34]. Overexpression of lncRNAs downregulate the corresponding miRNAs and further activates Wnt/β-catenin signaling, resulting in increased levels of Wnt target gene transcription and metastatic ability of HCC cells [46,47][33][34]. Additionally, upregulation of LINC00662 in HCC induced WNT3A secretion with miR-15a/16/107 binding, resulting in the activation of Wnt/β-catenin and polarizes M2 macrophage [48][35]. Similarly, overexpressing FEZF1-AS1 negatively regulates the level of miR-107, which inhibits the activation of Wnt/β-catenin signaling, while downregulation of FEZF1-AS1 enhances the expression of β-catenin [49][36]. Furthermore, both miR-122 and miR-148a were found to contribute to liver cancers by binding to the 3′-untranslated region (3′-UTR) site of Wnt1, suppressing the level of β-catenin and inhibiting Wnt-target gene transcription [50,51][37][38]. Furthermore, a decrease in these miRNA levels could cause excess Wnt/β-catenin signaling and increase EMT [50][37]. All the above mentioned enhance the progression of HCC. As a tumor suppressor, miR-34a is reported in mice and HCC patients and found to be upregulated through activated Wnt/β-catenin signaling [52][39]. In addition, overexpression of miR-145 has been shown to diminish the level of β-catenin, suppressing HCC cell growth [53][40]. To sum up, Wnt/β-catenin signaling is tightly regulated by DNA methylation, histone modification and non-coding RNAs in HCC (Figure 1).
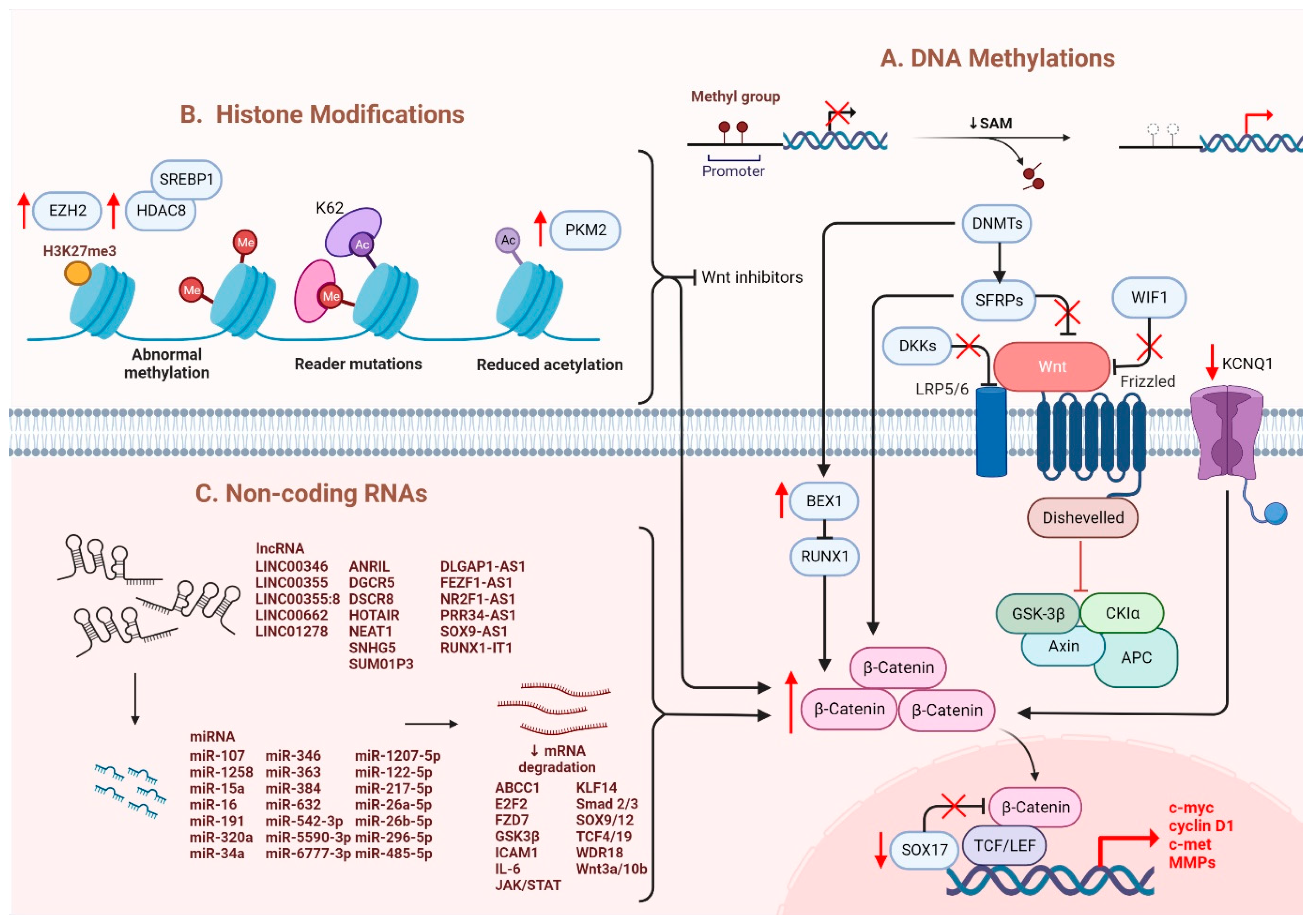
Figure 1. Regulation of Wnt/β-catenin signaling in HCC. Wnt/β-catenin signaling in HCC is regulated by (A) DNA methylation, (B) histone modification and (C) non-coding RNAs.
Table 1.
The list of lncRNAs and their related miRNAs in regulation of Wnt/β-catenin signaling in HCC.
| LncRNA | miRNA | Targets | Ref. |
|---|---|---|---|
| LINC00346 | miR-542-3p | FZD7, WDR18 | [54][41] |
| LINC00355 | miR-217-5p | GSK3β, c-myc, CCND1 | [46][33] |
| LINC00355:8 | miR-6777-3p | Wnt10b | [55][42] |
| LINC00662 | miR-15a, miR-16, miR-107 | Wnt3a | [48][35] |
| LINC01278 | miR-1258 | TCF-4, Smad2/3 | [47][34] |
| ANRIL | miR-191, miR-122-5p |
CCND1, p53, p21, MMP-2, MMP-9, Vimentin | [56,57][43][44] |
| DGCR5 | miR-346 | KLF14 | [58][45] |
| DSCR8 | miR-485-5p | FZD7 | [59][46] |
| DLGAP1-AS1 | miR-26a-5p, miR-26b-5p |
IL-6, JAK2, STAT3 | [60][47] |
| FEZF1-AS1 | miR-107 | Wnt3a, ICAM1, Vimentin | [49][36] |
| HOTAIR | miR-34a | Akt | [61][48] |
| MIR194-2HG | miR-1207-5p | TCF19 | [62][49] |
| NEAT1 | miR-384 | Wnt | [63][50] |
| NR2F1-AS1 | miR-363 | ABCC1 | [64][51] |
| PRR34-AS1 | miR-296-5p | E2F2, SOX12 | [65][52] |
| RUNX1-IT1 | miR-632 | GSK3β | [66][53] |
| SNHG5 | miR-26a-5p | GSK3β | [67][54] |
| SUMO1P3 | miR-320a | C-myc, CCND1 | [68][55] |
| SOX9-AS1 | miR-5590-3p | SOX9 | [69][56] |
2.3. Other Molecules Involved in the Regulation of Wnt/β-Catenin
Apart from genetic mutations and epigenetic dysregulation, other molecules/pathways were identified to regulate Wnt/β-catenin signaling. In normoric environment, ROS is maintained at a low level; whereas a steady increase of ROS level promotes cancer development and progression [70][57]. A recent study showed that Wnt/β-catenin signaling was suppressed upon elevation of intracellular ROS level [19,71][5][58]. In HCC, glutaminase 1 (GLS1) is upregulated which augmented liver CSC properties with increased expression of CSC markers via suppression of ROS level [19,71][5][58]. Likewise, another study also showed that ROS accumulation due to the overexpression of Cytochrome P450 2E1 (CYP2E1) decreased the activity of Wnt/β-catenin signaling through the degradation of DVL2 in HCC [72][59]. Hypoxia also plays a crucial role in the activation of Wnt/β-catenin signaling. Hypoxia-inducible factor 1-alpha (HIF1α), a hypoxia-inducible factor, regulates transcription in hypoxic environments and is also reported to mediate the expression of B-cell lymphoma 9 (BCL9) [9,73][16][60]. BCL9 can coactivate with HIF1α to enhance the transcriptional activity of β-catenin regardless of whether genetic mutations occur, resulting in activation of Wnt/β-catenin signaling and leading to HCC progression [73][60]. Furthermore, ZBTB20 has been reported in liver tumorigenesis with its role in suppressing PPARG expression and inhibiting proteasomal degradation of the β-catenin destruction complex [74][61]. Overall, once the nuclear translocation of β-catenin is achieved, the expression levels of the downstream genes involved in EMT are modulated and enhanced, causing hepatocarcinogenesis [17][3]. C-Myc is the most critical gene induced by activated Wnt/β-catenin signaling, which enhances the mechanisms of glycolysis and glutaminolysis [75][62]. This is followed by cyclin D1, which has been reported to be enhanced in both mouse and human HCC [76,77][63][64]. Specifically, overexpression of c-Met and cyclin D1 triggers the development of liver tumors and decreases survival in mice [78][65]. It is also noted that upregulation of cyclin D1 enhances tumor metastatic ability [79][66]. Additionally, studies have discovered that GS and VEGF are also involved in modulating the downstream effects of activated Wnt and assisting in angiogenesis [80][67], as the upregulation of multiple matrix metalloproteinases (MMPs), including MMP2 and MMP9, is associated with tumor metastasis [81][68]. Apart from gene regulation, aberrant β-catenin signaling also negatively regulates certain signaling cascades: for example, the suppression of NF-κB cascade in the liver [82][69]. Moreover, the crosstalk between Wnt and Hippo signaling pathways has been observed in HCC. Recent study showed that Wnt-Hippo signature related genes may be a potential markers for prediction of immune infiltration in HCC [83][70]. Notably, aberrant activation of β-catenin caused by the deletion of mammalian STE20-like protein kinase 1/2 (Mst1/2) promotes tumor growth, indicating the co-expression of YAP and β-catenin in HCC [84][71].
References
- He, S.; Tang, S. WNT/β-catenin signaling in the development of liver cancers. Biomed. Pharmacother. 2020, 132, 110851.
- Russell, J.O.; Monga, S.P. Wnt/β-Catenin Signaling in Liver Development, Homeostasis, and Pathobiology. Annu. Rev. Pathol. 2018, 13, 351–378.
- Xu, C.; Xu, Z.; Zhang, Y.; Evert, M.; Calvisi, D.F.; Chen, X. β-Catenin signaling in hepatocellular carcinoma. J. Clin. Investig. 2022, 132, e154515.
- Lu, L.C.; Shao, Y.Y.; Lee, Y.H.; Hsieh, M.S.; Hsiao, C.H.; Lin, H.H.; Kao, H.F.; Ma, Y.Y.; Yen, F.C.; Cheng, A.L.; et al. β-catenin (CTNNB1) mutations are not associated with prognosis in advanced hepatocellular carcinoma. Oncology 2014, 87, 159–166.
- Deldar Abad Paskeh, M.; Mirzaei, S.; Ashrafizadeh, M.; Zarrabi, A.; Sethi, G. Wnt/β-Catenin Signaling as a Driver of Hepatocellular Carcinoma Progression: An Emphasis on Molecular Pathways. J. Hepatocell. Carcinoma 2021, 8, 1415–1444.
- Monga, S.P. β-Catenin Signaling and Roles in Liver Homeostasis, Injury, and Tumorigenesis. Gastroenterology 2015, 148, 1294–1310.
- Javanmard, D.; Najafi, M.; Babaei, M.R.; Karbalaie Niya, M.H.; Esghaei, M.; Panahi, M.; Safarnezhad Tameshkel, F.; Tavakoli, A.; Jazayeri, S.M.; Ghaffari, H.; et al. Investigation of CTNNB1 gene mutations and expression in hepatocellular carcinoma and cirrhosis in association with hepatitis B virus infection. Infect. Agents Cancer 2020, 15, 37.
- Okabe, H.; Kinoshita, H.; Imai, K.; Nakagawa, S.; Higashi, T.; Arima, K.; Uchiyama, H.; Ikegami, T.; Harimoto, N.; Itoh, S.; et al. Diverse Basis of β-Catenin Activation in Human Hepatocellular Carcinoma: Implications in Biology and Prognosis. PLoS ONE 2016, 11, e0152695.
- Wong, C.M.; Fan, S.T.; Ng, I.O. beta-Catenin mutation and overexpression in hepatocellular carcinoma: Clinicopathologic and prognostic significance. Cancer 2001, 92, 136–145.
- Wang, W.; Pan, Q.; Fuhler, G.M.; Smits, R.; Peppelenbosch, M.P. Action and function of Wnt/β-catenin signaling in the progression from chronic hepatitis C to hepatocellular carcinoma. J. Gastroenterol. 2017, 52, 419–431.
- Liu, P.; Liang, B.; Liu, M.; Lebbink, J.H.G.; Li, S.; Qian, M.; Lavrijsen, M.; Peppelenbosch, M.P.; Chen, X.; Smits, R. Oncogenic Mutations in Armadillo Repeats 5 and 6 of β-Catenin Reduce Binding to APC, Increasing Signaling and Transcription of Target Genes. Gastroenterology 2020, 158, 1029–1043.e10.
- Kim, E.; Lisby, A.; Ma, C.; Lo, N.; Ehmer, U.; Hayer, K.E.; Furth, E.E.; Viatour, P. Promotion of growth factor signaling as a critical function of β-catenin during HCC progression. Nat. Commun. 2019, 10, 1909.
- Kim, S.; Jeong, S. Mutation Hotspots in the β-Catenin Gene: Lessons from the Human Cancer Genome Databases. Mol. Cells 2019, 42, 8–16.
- Harada, N.; Miyoshi, H.; Murai, N.; Oshima, H.; Tamai, Y.; Oshima, M.; Taketo, M.M. Lack of tumorigenesis in the mouse liver after adenovirus-mediated expression of a dominant stable mutant of beta-catenin. Cancer Res. 2002, 62, 1971–1977.
- Liu, L.J.; Xie, S.X.; Chen, Y.T.; Xue, J.L.; Zhang, C.J.; Zhu, F. Aberrant regulation of Wnt signaling in hepatocellular carcinoma. World J. Gastroenterol. 2016, 22, 7486–7499.
- Khalaf, A.M.; Fuentes, D.; Morshid, A.I.; Burke, M.R.; Kaseb, A.O.; Hassan, M.; Hazle, J.D.; Elsayes, K.M. Role of Wnt/β-catenin signaling in hepatocellular carcinoma, pathogenesis, and clinical significance. J. Hepatocell. Carcinoma 2018, 5, 61–73.
- Inagawa, S.; Itabashi, M.; Adachi, S.; Kawamoto, T.; Hori, M.; Shimazaki, J.; Yoshimi, F.; Fukao, K. Expression and prognostic roles of beta-catenin in hepatocellular carcinoma: Correlation with tumor progression and postoperative survival. Clin. Cancer Res. 2002, 8, 450–456.
- Zucman-Rossi, J.; Benhamouche, S.; Godard, C.; Boyault, S.; Grimber, G.; Balabaud, C.; Cunha, A.S.; Bioulac-Sage, P.; Perret, C. Differential effects of inactivated Axin1 and activated beta-catenin mutations in human hepatocellular carcinomas. Oncogene 2007, 26, 774–780.
- Raggi, C.; Factor, V.M.; Seo, D.; Holczbauer, A.; Gillen, M.C.; Marquardt, J.U.; Andersen, J.B.; Durkin, M.; Thorgeirsson, S.S. Epigenetic reprogramming modulates malignant properties of human liver cancer. Hepatology 2014, 59, 2251–2262.
- Wang, Q.; Liang, N.; Yang, T.; Li, Y.; Li, J.; Huang, Q.; Wu, C.; Sun, L.; Zhou, X.; Cheng, X.; et al. DNMT1-mediated methylation of BEX1 regulates stemness and tumorigenicity in liver cancer. J. Hepatol. 2021, 75, 1142–1153.
- Lin, Y.W.; Shih, Y.L.; Lien, G.S.; Suk, F.M.; Hsieh, C.B.; Yan, M.D. Promoter methylation of SFRP3 is frequent in hepatocellular carcinoma. Dis. Markers 2014, 2014, 351863.
- Xie, Q.; Chen, L.; Shan, X.; Shan, X.; Tang, J.; Zhou, F.; Chen, Q.; Quan, H.; Nie, D.; Zhang, W.; et al. Epigenetic silencing of SFRP1 and SFRP5 by hepatitis B virus X protein enhances hepatoma cell tumorigenicity through Wnt signaling pathway. Int. J. Cancer 2014, 135, 635–646.
- Takagi, H.; Sasaki, S.; Suzuki, H.; Toyota, M.; Maruyama, R.; Nojima, M.; Yamamoto, H.; Omata, M.; Tokino, T.; Imai, K.; et al. Frequent epigenetic inactivation of SFRP genes in hepatocellular carcinoma. J. Gastroenterol. 2008, 43, 378–389.
- Ding, Z.; Qian, Y.B.; Zhu, L.X.; Xiong, Q.R. Promoter methylation and mRNA expression of DKK-3 and WIF-1 in hepatocellular carcinoma. World J. Gastroenterol. 2009, 15, 2595–2601.
- Jia, Y.; Yang, Y.; Liu, S.; Herman, J.G.; Lu, F.; Guo, M. SOX17 antagonizes WNT/β-catenin signaling pathway in hepatocellular carcinoma. Epigenetics 2010, 5, 743–749.
- Tian, Y.; Mok, M.T.; Yang, P.; Cheng, A.S. Epigenetic Activation of Wnt/β-Catenin Signaling in NAFLD-Associated Hepatocarcinogenesis. Cancers 2016, 8, 76.
- Fan, H.; Zhang, M.; Liu, W. Hypermethylated KCNQ1 acts as a tumor suppressor in hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2018, 503, 3100–3107.
- Song, H.; Yu, Z.; Sun, X.; Feng, J.; Yu, Q.; Khan, H.; Zhu, X.; Huang, L.; Li, M.; Mok, M.T.S.; et al. Androgen receptor drives hepatocellular carcinogenesis by activating enhancer of zeste homolog 2-mediated Wnt/β-catenin signaling. EBioMedicine 2018, 35, 155–166.
- Cheng, A.S.; Lau, S.S.; Chen, Y.; Kondo, Y.; Li, M.S.; Feng, H.; Ching, A.K.; Cheung, K.F.; Wong, H.K.; Tong, J.H.; et al. EZH2-mediated concordant repression of Wnt antagonists promotes β-catenin-dependent hepatocarcinogenesis. Cancer Res. 2011, 71, 4028–4039.
- Tian, Y.; Wong, V.W.; Wong, G.L.; Yang, W.; Sun, H.; Shen, J.; Tong, J.H.; Go, M.Y.; Cheung, Y.S.; Lai, P.B.; et al. Histone Deacetylase HDAC8 Promotes Insulin Resistance and β-Catenin Activation in NAFLD-Associated Hepatocellular Carcinoma. Cancer Res. 2015, 75, 4803–4816.
- Zhang, R.; Shen, M.; Wu, C.; Chen, Y.; Lu, J.; Li, J.; Zhao, L.; Meng, H.; Zhou, X.; Huang, G.; et al. HDAC8-dependent deacetylation of PKM2 directs nuclear localization and glycolysis to promote proliferation in hepatocellular carcinoma. Cell Death Dis. 2020, 11, 1036.
- Shi, T.; Morishita, A.; Kobara, H.; Masaki, T. The Role of Long Non-Coding RNA and microRNA Networks in Hepatocellular Carcinoma and Its Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 630.
- Luo, X.; ABudureyimu, M.; Yang, G.; Yan, Z.; Fu, X.; Lu, P.; Zhang, D.; Zhang, S.; Ding, Z. LINC00355 triggers malignant progression of hepatocellular carcinoma via the sponge effect on miR-217-5p with the involvement of the Wnt/β-catenin signaling. J. BUON 2021, 26, 1964–1969.
- Huang, W.J.; Tian, X.P.; Bi, S.X.; Zhang, S.R.; He, T.S.; Song, L.Y.; Yun, J.P.; Zhou, Z.G.; Yu, R.M.; Li, M. The β-catenin/TCF-4-LINC01278-miR-1258-Smad2/3 axis promotes hepatocellular carcinoma metastasis. Oncogene 2020, 39, 4538–4550.
- Tian, X.; Wu, Y.; Yang, Y.; Wang, J.; Niu, M.; Gao, S.; Qin, T.; Bao, D. Long noncoding RNA LINC00662 promotes M2 macrophage polarization and hepatocellular carcinoma progression via activating Wnt/β-catenin signaling. Mol. Oncol. 2020, 14, 462–483.
- Yao, J.; Yang, Z.; Yang, J.; Wang, Z.G.; Zhang, Z.Y. Long non-coding RNA FEZF1-AS1 promotes the proliferation and metastasis of hepatocellular carcinoma via targeting miR-107/Wnt/β-catenin axis. Aging 2021, 13, 13726–13738.
- Yan, H.; Dong, X.; Zhong, X.; Ye, J.; Zhou, Y.; Yang, X.; Shen, J.; Zhang, J. Inhibitions of epithelial to mesenchymal transition and cancer stem cells-like properties are involved in miR-148a-mediated anti-metastasis of hepatocellular carcinoma. Mol. Carcinog. 2014, 53, 960–969.
- Xu, J.; Zhu, X.; Wu, L.; Yang, R.; Yang, Z.; Wang, Q.; Wu, F. MicroRNA-122 suppresses cell proliferation and induces cell apoptosis in hepatocellular carcinoma by directly targeting Wnt/β-catenin pathway. Liver Int. 2012, 32, 752–760.
- Gougelet, A.; Sartor, C.; Bachelot, L.; Godard, C.; Marchiol, C.; Renault, G.; Tores, F.; Nitschke, P.; Cavard, C.; Terris, B.; et al. Antitumour activity of an inhibitor of miR-34a in liver cancer with β-catenin-mutations. Gut 2016, 65, 1024–1034.
- Jin, X.; Chen, Y.P.; Kong, M.; Zheng, L.; Yang, Y.D.; Li, Y.M. Transition from hepatic steatosis to steatohepatitis: Unique microRNA patterns and potential downstream functions and pathways. J. Gastroenterol. Hepatol. 2012, 27, 331–340.
- Zhang, N.; Chen, X. A positive feedback loop involving the LINC00346/β-catenin/MYC axis promotes hepatocellular carcinoma development. Cell Oncol. 2020, 43, 137–153.
- Zhou, F.; Lei, Y.; Xu, X.; Zhou, H.; Liu, H.; Jiang, J.; Yang, Y.; Wu, B. LINC00355:8 promotes cell proliferation and migration with invasion via the MiR-6777-3p/Wnt10b axis in Hepatocellular Carcinoma. J. Cancer 2020, 11, 5641–5655.
- Huang, D.; Bi, C.; Zhao, Q.; Ding, X.; Bian, C.; Wang, H.; Wang, T.; Liu, H. Knockdown long non-coding RNA ANRIL inhibits proliferation, migration and invasion of HepG2 cells by down-regulation of miR-191. BMC Cancer 2018, 18, 919.
- Ma, J.; Li, T.; Han, X.; Yuan, H. Knockdown of LncRNA ANRIL suppresses cell proliferation, metastasis, and invasion via regulating miR-122-5p expression in hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2018, 144, 205–214.
- Wang, Y.G.; Liu, J.; Shi, M.; Chen, F.X. LncRNA DGCR5 represses the development of hepatocellular carcinoma by targeting the miR-346/KLF14 axis. J. Cell. Physiol. 2018, 234, 572–580.
- Wang, Y.; Sun, L.; Wang, L.; Liu, Z.; Li, Q.; Yao, B.; Wang, C.; Chen, T.; Tu, K.; Liu, Q. Long non-coding RNA DSCR8 acts as a molecular sponge for miR-485-5p to activate Wnt/β-catenin signal pathway in hepatocellular carcinoma. Cell Death Dis. 2018, 9, 851.
- Lin, Y.; Jian, Z.; Jin, H.; Wei, X.; Zou, X.; Guan, R.; Huang, J. Long non-coding RNA DLGAP1-AS1 facilitates tumorigenesis and epithelial-mesenchymal transition in hepatocellular carcinoma via the feedback loop of miR-26a/b-5p/IL-6/JAK2/STAT3 and Wnt/β-catenin pathway. Cell Death Dis. 2020, 11, 34.
- Duan, Y.; Chen, J.; Yang, Y.; Qu, Z.; Lu, Y.; Sun, D. LncRNA HOTAIR contributes Taxol-resistance of hepatocellular carcinoma cells via activating AKT phosphorylation by down-regulating miR-34a. Biosci. Rep. 2020, 40, BSR20201627.
- Xu, G.; Zhu, Y.; Liu, H.; Liu, Y.; Zhang, X. LncRNA MIR194-2HG Promotes Cell Proliferation and Metastasis via Regulation of miR-1207-5p/TCF19/Wnt/β-Catenin Signaling in Liver Cancer. Onco. Targets Ther. 2020, 13, 9887–9899.
- Zhu, L.; Yang, N.; Li, C.; Liu, G.; Pan, W.; Li, X. Long noncoding RNA NEAT1 promotes cell proliferation, migration, and invasion in hepatocellular carcinoma through interacting with miR-384. J. Cell. Biochem. 2018, 120, 1997–2006.
- Huang, H.; Chen, J.; Ding, C.M.; Jin, X.; Jia, Z.M.; Peng, J. LncRNA NR2F1-AS1 regulates hepatocellular carcinoma oxaliplatin resistance by targeting ABCC1 via miR-363. J. Cell. Mol. Med. 2018, 22, 3238–3245.
- Qin, M.; Meng, Y.; Luo, C.; He, S.; Qin, F.; Yin, Y.; Huang, J.; Zhao, H.; Hu, J.; Deng, Z.; et al. lncRNA PRR34-AS1 promotes HCC development via modulating Wnt/β-catenin pathway by absorbing miR-296-5p and upregulating E2F2 and SOX12. Mol. Ther. Nucleic Acids 2021, 25, 37–52.
- Sun, L.; Wang, L.; Chen, T.; Shi, Y.; Yao, B.; Liu, Z.; Wang, Y.; Li, Q.; Liu, R.; Niu, Y.; et al. LncRNA RUNX1-IT1 which is downregulated by hypoxia-driven histone deacetylase 3 represses proliferation and cancer stem-like properties in hepatocellular carcinoma cells. Cell Death Dis. 2020, 11, 95.
- Li, Y.; Guo, D.; Zhao, Y.; Ren, M.; Lu, G.; Wang, Y.; Zhang, J.; Mi, C.; He, S.; Lu, X. Long non-coding RNA SNHG5 promotes human hepatocellular carcinoma progression by regulating miR-26a-5p/GSK3β signal pathway. Cell Death Dis. 2018, 9, 888.
- Wu, S.; Chen, S.; Lin, N.; Yang, J. Long non-coding RNA SUMO1P3 promotes hepatocellular carcinoma progression through activating Wnt/β-catenin signalling pathway by targeting miR-320a. J. Cell. Mol. Med. 2020, 24, 3108–3116.
- Zhang, W.; Wu, Y.; Hou, B.; Wang, Y.; Deng, D.; Fu, Z.; Xu, Z. A SOX9-AS1/miR-5590-3p/SOX9 positive feedback loop drives tumor growth and metastasis in hepatocellular carcinoma through the Wnt/β-catenin pathway. Mol. Oncol. 2019, 13, 2194–2210.
- Aggarwal, V.; Tuli, H.S.; Varol, A.; Thakral, F.; Yerer, M.B.; Sak, K.; Varol, M.; Jain, A.; Khan, M.A.; Sethi, G. Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules 2019, 9, 735.
- Li, B.; Cao, Y.; Meng, G.; Qian, L.; Xu, T.; Yan, C.; Luo, O.; Wang, S.; Wei, J.; Ding, Y.; et al. Targeting glutaminase 1 attenuates stemness properties in hepatocellular carcinoma by increasing reactive oxygen species and suppressing Wnt/beta-catenin pathway. EBioMedicine 2019, 39, 239–254.
- Zhu, L.; Yang, X.; Feng, J.; Mao, J.; Zhang, Q.; He, M.; Mi, Y.; Mei, Y.; Jin, G.; Zhang, H. CYP2E1 plays a suppressive role in hepatocellular carcinoma by regulating Wnt/Dvl2/β-catenin signaling. J. Transl. Med. 2022, 20, 194.
- Xu, W.; Zhou, W.; Cheng, M.; Wang, J.; Liu, Z.; He, S.; Luo, X.; Huang, W.; Chen, T.; Yan, W.; et al. Hypoxia activates Wnt/β-catenin signaling by regulating the expression of BCL9 in human hepatocellular carcinoma. Sci. Rep. 2017, 7, 40446.
- To, J.C.; Chiu, A.P.; Tschida, B.R.; Lo, L.H.; Chiu, C.H.; Li, X.X.; Kuka, T.P.; Linden, M.A.; Amin, K.; Chan, W.C.; et al. ZBTB20 regulates WNT/CTNNB1 signalling pathway by suppressing PPARG during hepatocellular carcinoma tumourigenesis. JHEP Rep. 2021, 3, 100223.
- Liu, R.; Li, Y.; Tian, L.; Shi, H.; Wang, J.; Liang, Y.; Sun, B.; Wang, S.; Zhou, M.; Wu, L.; et al. Gankyrin drives metabolic reprogramming to promote tumorigenesis, metastasis and drug resistance through activating β-catenin/c-Myc signaling in human hepatocellular carcinoma. Cancer Lett. 2019, 443, 34–46.
- Delgado, E.; Bahal, R.; Yang, J.; Lee, J.M.; Ly, D.H.; Monga, S.P. β-Catenin knockdown in liver tumor cells by a cell permeable gamma guanidine-based peptide nucleic acid. Curr. Cancer Drug Targets 2013, 13, 867–878.
- Kaur, P.; Mani, S.; Cros, M.P.; Scoazec, J.Y.; Chemin, I.; Hainaut, P.; Herceg, Z. Epigenetic silencing of sFRP1 activates the canonical Wnt pathway and contributes to increased cell growth and proliferation in hepatocellular carcinoma. Tumour Biol. 2012, 33, 325–336.
- Patil, M.A.; Lee, S.A.; Macias, E.; Lam, E.T.; Xu, C.; Jones, K.D.; Ho, C.; Rodriguez-Puebla, M.; Chen, X. Role of cyclin D1 as a mediator of c-Met- and beta-catenin-induced hepatocarcinogenesis. Cancer Res. 2009, 69, 253–261.
- Tang, B.; Tang, F.; Wang, Z.; Qi, G.; Liang, X.; Li, B.; Yuan, S.; Liu, J.; Yu, S.; He, S. Overexpression of CTNND1 in hepatocellular carcinoma promotes carcinous characters through activation of Wnt/β-catenin signaling. J. Exp. Clin. Cancer Res. 2016, 35, 82.
- Qu, B.; Liu, B.R.; Du, Y.J.; Chen, J.; Cheng, Y.Q.; Xu, W.; Wang, X.H. Wnt/β-catenin signaling pathway may regulate the expression of angiogenic growth factors in hepatocellular carcinoma. Oncol. Lett. 2014, 7, 1175–1178.
- Wang, C.; Zhang, R.; Wang, X.; Zheng, Y.; Jia, H.; Li, H.; Wang, J.; Wang, N.; Xiang, F.; Li, Y. Silencing of KIF3B Suppresses Breast Cancer Progression by Regulating EMT and Wnt/β-Catenin Signaling. Front. Oncol. 2020, 10, 597464.
- Ma, B.; Hottiger, M.O. Crosstalk between Wnt/β-Catenin and NF-κB Signaling Pathway during Inflammation. Front. Immunol. 2016, 7, 378.
- Chang, Y.S.; Chou, Y.P.; Chung, C.C.; Lee, Y.T.; Yen, J.C.; Jeng, L.B.; Chang, J.G. Molecular Classification of Hepatocellular Carcinoma Using Wnt–Hippo Signaling Pathway-Related Genes. Cancers 2022, 14, 4580.
- Song, H.; Mak, K.K.; Topol, L.; Yun, K.; Hu, J.; Garrett, L.; Chen, Y.; Park, O.; Chang, J.; Simpson, R.M.; et al. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc. Natl. Acad. Sci. USA 2010, 107, 1431–1436.
More
