Cells, the basic structures of all living organisms, reside in an extracellular matrix consisting of a complex three-dimensional architecture and interact with neighboring cells both mechanically and biochemically. Cell–cell and cell–extracellular matrix interactions form a three-dimensional network that maintains tissue specificity and homeostasis. Important biological processes in a cell cycle are regulated by principles organized by the microenvironment surrounding the cell. The conventional cell culture methods failed to mimic in vivo-like structural organization and are insufficient to examine features such as connectivity of cells, cellular morphology, viability, proliferation, differentiation, gene and protein expression, response to stimuli, and drug/vaccine metabolism. Three-dimensional cell culture studies are very important in terms of reducing the need for in vivo studies and creating an intermediate step.
- cell culture
- infectious disease
- three-dimensional
- vaccine
- pathogen-host interaction
1. Introduction
2. Three-Dimensional Cell Culture Methods
Cells, the building blocks of tissues and organs in the organism, reside in a complex 3D extracellular matrix (ECM) environment. This complex 3D architecture allows cells to interact both with each other and with the ECM. In this way, each cell acquires its own morphology and maintains the specificity and homeostasis of the tissue. Today, new methods have been developed that enable cells to grow in this complex 3D architecture in a laboratory environment. These methods are basically divided into two categories: scaffold-free methods and scaffold-based methods (Figure 1).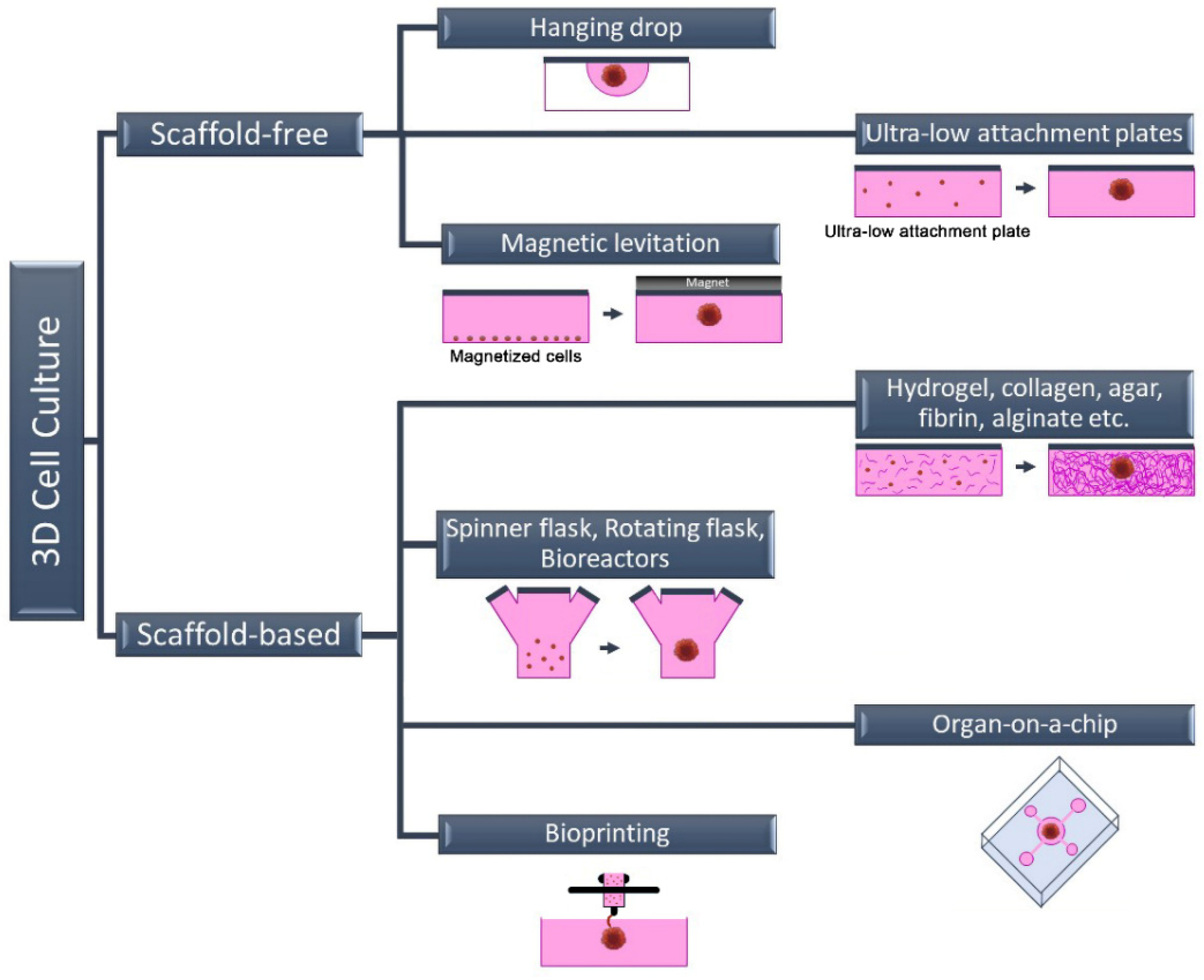
2.1. Scaffold-Free 3D Cell Culture Method
The most commonly used scaffold-free 3D cell culture techniques are cell suspension culture on non-adhesive or ultra-low attachment plates (liquid overlay), hanging drop, magnetic levitation, and microfluidic. Scaffold-free methods are generally fast and economical. This method is a “bottom-up” approach and is based on the fact that cells come together to form a spheroid structure. The formation of 3D spheroids depends entirely on the natural abilities of the cells because there is no material that can be used as a scaffold in these techniques. The hanging drop technique gravitationally collects cells in the form of suspended drops at a spherical air–liquid interface, thus facilitating the formation of a 3D cell structure without a scaffold [16]. Similar to the cell suspension method, the cell density of the suspension can be varied depending on the desired cell aggregate size. Spheroid formation, in contrast to these approaches, can be accomplished through external physical intervention in some scaffold-free techniques such as magnetic levitation or agitation bioreactor. The biggest shortcoming of scaffold-free 3D cell culture methods is the absence of an ECM component. Since there is no ECM in the environment, only cell–cell interaction occurs. Cell–ECM interaction is missing, unlike in their natural environment [17]. The wells of the plates can also be coated with various chemicals, such as poly (2-hydroxyethyl methacrylate), agar, agarose, or pol (N-p vinyl benzyl-D-lactone amide), to produce a non-attached surface. However, this incurs additional equipment and/or costs. These approaches might not be adequate because they lack the scaffold support necessary for the cell–matrix interaction needed for cell biology and proper functioning [18,19][18][19].2.2. Scaffold-Based 3D Cell Culture Method
In scaffold-based 3D cell culture methods, ECM components are also added to the culture to provide extracellular components that mimic the biological environment. For this purpose, commercially available, ready-to-use scaffolds as well as suitable ECM components can be used. Unlike scaffold-free methods, the ECM is a complex and dynamic structure found between cells in these methods. In addition to providing structural support, ECM plays an active role in helping cells acquire tissue-specific properties. Although its content varies according to the characteristics of tissues and cells, ECM basically consists of two components: proteins (collagen, elastin, fibronectin, laminin, fibrillin, etc.) and proteoglycans (heparan sulfate, chondroitin sulfate, etc.) [20,21][20][21]. Each of these components varies according to the task undertaken by the cell, and they also change in different physiological and biochemical events such as proliferation, genetic changes, differentiation, attachment, and migration. Therefore, the ECM is defined as a dynamic structure. In scaffold-based 3D cell culture, natural polymers such as collagen, hydroxyapatite, agar, fibrin or alginate are used as scaffolds, as well as biodegradable synthetic polymers such as poly (ethylene glycol) and poly (lactide-co-glycolide) [20,21][20][21]. In addition to homologous 3D cell culture, heterologous 3D spheroids and organoids can be prepared by scaffold-based methods. Organoids called “Culturable Mini-Organs” are thought of as miniature versions of organs. On the other hand, spheroids can be prepared either homogeneously (from a single cell type) or heterogeneously (from different cell types) (Figure 2). Mostly, immortal cell lines are used for spheroids, while organoids are prepared from adult or embryonic stem cells. For this reason, organoids are complex structures that can better mimic organs [22,23][22][23]. On the contrary, spheroids are more economical and easy-to-prepare structures that can also be called “cell aggregates” or “organotypic culture” [24,25][24][25]. Although both approaches are used in vaccine and drug research and in in vitro disease modeling, spheroids are frequently preferred in tumor and drug development studies. Organoids are preferred in vaccine and pathogen interaction studies for assessing immune responses, especially in mimicking complex and multi-component organs such as the respiratory tract. Heterologous 3D models, in which more than one cell type is cultured together, also provide cell–cell interactions where growth factors and other biological factors can be exchanged [26,27][26][27]. The interactions of cells with each other and with the ECM are very important in terms of cell polarity. Cell polarity plays a direct role in the viral–host relationship as it affects the expression of the relevant receptor. For this reason, heterologous 3D cell culture studies attract attention as an effective in vitro study method in infection and virology studies.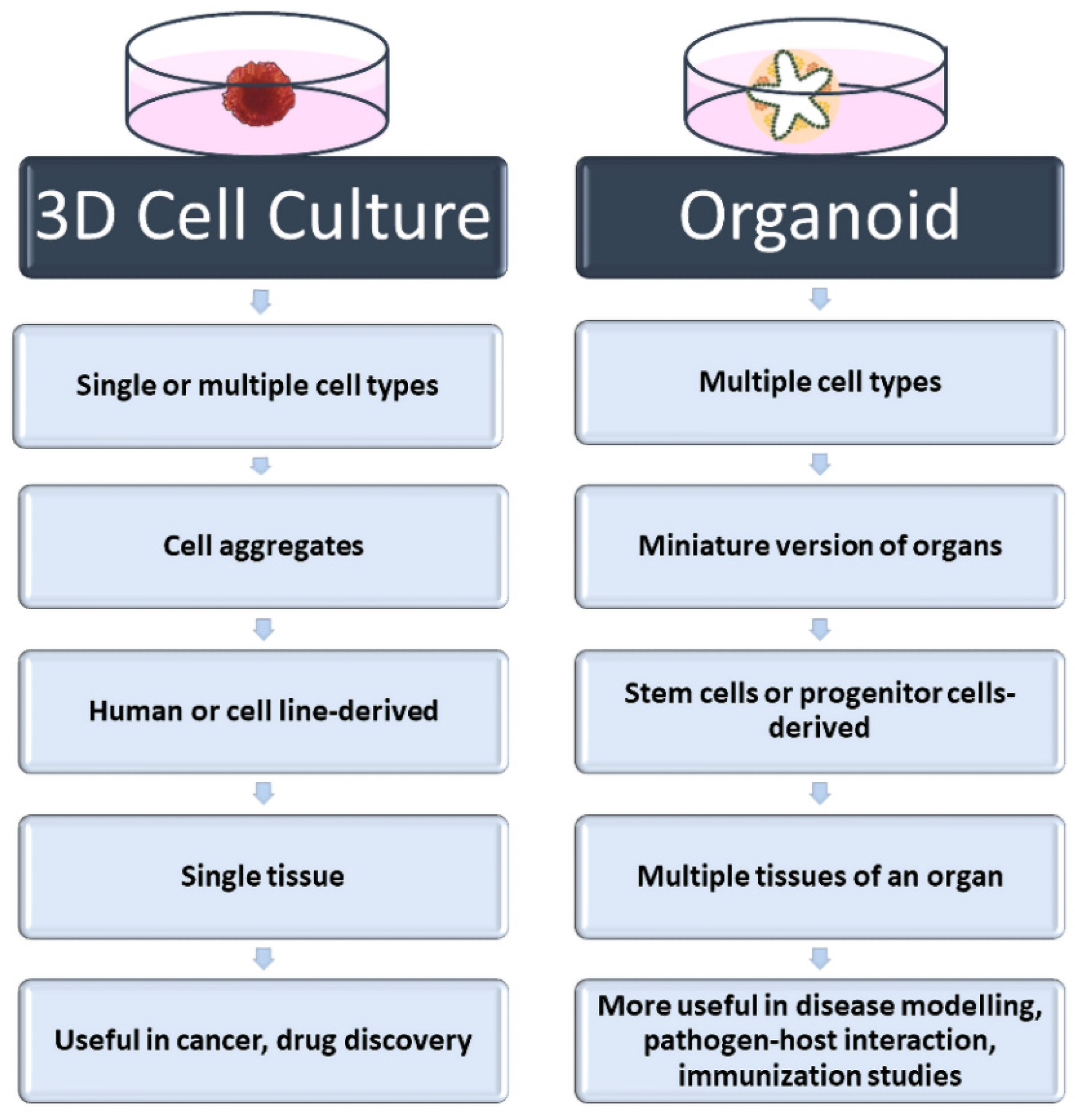
References
- Dolskiy, A.A.; Grishchenko, I.V.; Yudkin, D.V. Cell Cultures for Virology: Usability, Advantages, and Prospects. Int. J. Mol. Sci. 2020, 21, 7978.
- Han, F.; Wang, J.; Ding, L.; Hu, Y.; Li, W.; Yuan, Z.; Guo, Q.; Zhu, C.; Yu, L.; Wang, H.; et al. Tissue Engineering and Regenerative Medicine: Achievements, Future, and Sustainability in Asia. Front. Bioeng. Biotechnol. 2020, 8, 83.
- Hudu, S.A.; Alshrari, A.S.; Syahida, A.; Sekawi, Z. Cell Culture, Technology: Enhancing the Culture of Diagnosing Human Diseases. J. Clin. Diagn. Res. 2016, 10, DE01–DE05.
- Verma, A.; Verma, M.; Singh, A. Animal tissue culture principles and applications. In Animal Biotechnology; Academic Press: Cambridge, MS, USA, 2020; pp. 269–293.
- O’Flaherty, R.; Bergin, A.; Flampouri, E.; Mota, L.M.; Obaidi, I.; Quigley, A.; Xie, Y.; Butler, M. Mammalian cell culture for production of recombinant proteins: A review of the critical steps in their biomanufacturing. Biotechnol. Adv. 2020, 43, 107552.
- Ju, X.; Zhu, Y.; Wang, Y.; Li, J.; Zhang, J.; Gong, M.; Ren, W.; Li, S.; Zhong, J.; Zhang, L.; et al. A novel cell culture system modeling the SARS-CoV-2 life cycle. PLoS Pathog. 2021, 17, e1009439.
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734.
- Graham, B.S. Advances in antiviral vaccine development. Immunol. Rev. 2013, 255, 230–242.
- Josefsberg, J.O.; Buckland, B. Vaccine process technology. Biotechnol. Bioeng. 2012, 109, 1443–1460.
- Cacciamali, A.; Villa, R.; Dotti, S. 3D Cell Cultures: Evolution of an Ancient Tool for New Applications. Front. Physiol. 2022, 13, 836480.
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33.
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures - a comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919.
- Barrila, J.; Crabbé, A.; Yang, J.; Franco, K.; Nydam, S.D.; Forsyth, R.J.; Davis, R.R.; Gangaraju, S.; Ott, C.M.; Coyne, C.B.; et al. Modeling Host-Pathogen Interactions in the Context of the Microenvironment: Three-Dimensional Cell Culture Comes of Age. Infect. Immun. 2018, 86, e00282-18.
- Häfner, S.J. Level up for culture models - How 3D cell culture models benefit SARS-CoV-2 research. Biomed. J. 2021, 44, 1–6.
- Harb, A.; Fakhreddine, M.; Zaraket, H.; Saleh, F.A. Three-Dimensional Cell Culture Models to Study Respiratory Virus Infections Including COVID-19. Biomimetics 2021, 7, 3.
- Hsiao, A.Y.; Tung, Y.C.; Qu, X.; Patel, L.R.; Pienta, K.J.; Takayama, S. 384 hanging drop arrays give excellent Z-factors and allow versatile formation of co-culture spheroids. Biotechnol. Bioeng. 2012, 109, 1293–1304.
- Alghuwainem, A.; Alshareeda, A.T.; Alsowayan, B. Scaffold-Free 3-D Cell Sheet Technique Bridges the Gap between 2-D Cell Culture and Animal Models. Int. J. Mol. Sci. 2019, 20, 4926.
- Foty, R. A simple hanging drop cell culture protocol for generation of 3D spheroids. J. Vis. Exp. 2011, 51, e2720.
- Shri, M.; Agrawal, H.; Rani, P.; Singh, D.; Onteru, S.K. Hanging Drop, A Best Three-Dimensional (3D) Culture Method for Primary Buffalo and Sheep Hepatocytes. Sci. Rep. 2017, 7, 1203.
- Hu, M.; Ling, Z.; Ren, X. Extracellular matrix dynamics: Tracking in biological systems and their implications. J. Biol. Eng. 2022, 16, 13.
- Nicolas, J.; Magli, S.; Rabbachin, L.; Sampaolesi, S.; Nicotra, F.; Russo, L. 3D Extracellular Matrix Mimics: Fundamental Concepts and Role of Materials Chemistry to Influence Stem Cell Fate. Biomacromolecules 2020, 21, 1968–1994.
- Koike, H.; Takebe, T. Generating Mini-Organs in Culture. Curr. Pathobiol. Rep. 2016, 4, 59–68.
- Dutta, D.; Clevers, H. Organoid culture systems to study host-pathogen interactions. Curr. Opin. Immunol. 2017, 48, 15–22.
- Gunti, S.; Hoke, A.T.K.; Vu, K.P.; London, N.R., Jr. Organoid and Spheroid Tumor Models: Techniques and Applications. Cancers 2021, 13, 874.
- Zanoni, M.; Cortesi, M.; Zamagni, A.; Arienti, C.; Pignatta, S.; Tesei, A. Modeling neoplastic disease with spheroids and organoids. J. Hematol. Oncol. 2020, 13, 97.
- Altmann, B.; Grün, C.; Nies, C.; Gottwald, E. Advanced 3D Cell Culture Techniques in Micro-Bioreactors, Part II: Systems and Applications. Processes 2021, 9, 21.
- Sangeeta, B.; Ankita Jaywant, D.; Shafina, S.; Jyotirmoi, A.; Soumya, B. Two-Dimensional and Three-Dimensional Cell Culture and Their Applications, in Cell Culture; Zhan, X., Ed.; IntechOpen: Rijeka, Croatia, 2021.
- Dey, M.; Ozbolat, I.T. 3D bioprinting of cells, tissues and organs. Sci. Rep. 2020, 10, 14023.
- Kabir, A.; Datta, P.; Oh, J.; Williams, A.; Ozbolat, V.; Unutmaz, D.; T Ozbolat, I. 3D Bioprinting for fabrication of tissue models of COVID-19 infection. Essays Biochem. 2021, 65, 503–518.
- Koban, R.; Lam, T.; Schwarz, F.; Kloke, L.; Bürge, S.; Ellerbrok, H.; Neumann, M. Simplified Bioprinting-Based 3D Cell Culture Infection Models for Virus Detection. Viruses 2020, 12, 1298.
- Grün, C.; Altmann, B.; Gottwald, E. Advanced 3D Cell Culture Techniques in Micro-Bioreactors, Part I: A Systematic Analysis of the Literature Published between 2000 and 2020. Processes 2020, 8, 1656.
- Kizilova, N.; Rokicki, J. 3D Bioreactors for Cell Culture: Fluid Dynamics Aspects. In Biomechanics in Medicine, Sport and Biology; Springer International Publishing: Cham, Switzerland, 2022.
- Yi, T.; Huang, S.; Liu, G.; Li, T.; Kang, Y.; Luo, Y.; Wu, J. Bioreactor Synergy with 3D Scaffolds: New Era for Stem Cells Culture. ACS Appl. Bio. Mater. 2018, 1, 193–209.
- Ingber, D.E. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet. 2022, 23, 467–491.
- Sun, W.; Luo, Z.; Lee, J.; Kim, H.-J.; Lee, K.; Tebon, P.; Feng, Y.; Dokmeci, M.R.; Sengupta, S.; Khademhosseini, A. Organ-on-a-Chip for Cancer and Immune Organs Modeling. Adv. Healthc. Mater. 2019, 8, 1801363.
- Tang, H.; Abouleila, Y.; Si, L.; Ortega-Prieto, A.M.; Mummery, C.L.; Ingber, D.E.; Mashaghi, A. Human Organs-on-Chips for Virology. Trends Microbiol. 2020, 28, 934–946.
- Wang, Y.; Wang, P.; Qin, J. Human Organoids and Organs-on-Chips for Addressing COVID-19 Challenges. Adv. Sci. 2022, 9, 2105187.
