The scale of research for developing and applying silicon carbide (SiC) membranes for gas separation has rapidly expanded over the last few decades. The precursor-derived ceramic approaches for preparing SiC membranes include chemical vapor deposition (CVD)/chemical vapor infiltration (CVI) deposition and pyrolysis of polymeric precursor. Generally, SiC membranes formed using the CVD/CVI deposition route have dense structures, making such membranes suitable for small-molecule gas separation. On the contrary, pyrolysis of a polymeric precursor is the most common and promising route for preparing SiC membranes, which includes the steps of precursor selection, coating/shaping, curing for cross-linking, and pyrolysis. Among these steps, the precursor, curing method, and pyrolysis temperature significantly impact the final microstructures and separation performance of membranes. Ref.
- silicon carbide membrane
- gas separation
- precursor-derived ceramics
- polycarbosilane
- allyl-hydridopolycarbosilan
- CVD/CVI
- pyrolysis
- organic to inorganic transformation
1. Introduction
1.1. Membrane-Based Gas Separation
1.2. Inorganic Membranes
| Membrane Material | Amorphous Structure | Advantages | Disadvantages |
|---|---|---|---|
| Silica (SiO2) |  |
|
|
| Carbon molecular sieve (CMS) or carbon membranes |
 [12] [12] |
|
|
| Silicon carbide (SiC) |  |
|
|
2. Fabrication of SiC Membranes
SiC membranes can be divided according to their morphological construction into symmetrical and asymmetrical types, as shown in Figure 12 [13][38]. Relatively high permeation fluxes can be obtained via asymmetric membranes, which consist of a support, transition layers (e.g., a particle layer and/or an intermediate layer), and a separation layer. The supports without separation layers, also known as membrane substrates, play an important role in producing defect-free and reproducible separation layers, which in turn play an important role in meeting industrial demand. The membrane substrates should have good characteristics to provide high flux, porosity, and mechanical strength. Currently, materials used as substrates are mainly alumina, mullite, titania, zirconia, SiC, and their composites [14][15][35,39]. Among them, SiC is a promising material for fabricating membrane substrates due to its unique properties. Generally, the same SiC substrates can be used as membrane filters, e.g., for microfiltration (MF, 0.05–10 μm), ultrafiltration (UF, 2–50 nm), and nanofiltration (NF, ≤2 nm) [16][17][18][37,40,41]. SiC supports/filters with large pores (>10 μm) are commonly used for air filtering, soot filtering, and catalyst distributors in diesel engines [16][19][37,42]. Processing methods for these supports/filters, such as extrusion [20][43], tape casting [21][22][44,45], slip-casting [23][46], and compression molding [24][47], have been extensively investigated.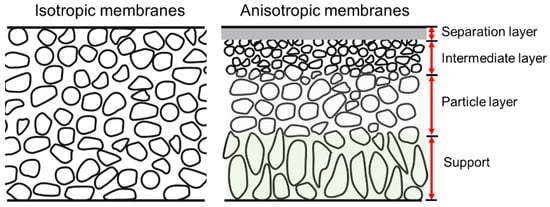
| Shape and Pore Size of Supports | Raw Materials of Layers on Supports | Layer Deposition Method | Top Layer Thickness [μm] | Thermal Treatment | Pore Size (Type: MF, UF) | Applications and Other Remarks | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Transition Layer | Top Layer | |||||||
| Disk, 15 μm | na. | α-SiC powder (10 μm), SiC whisker, methylcellulose (MC) 2, CaO 5, ZrO2 5, mullite 5, TL-56NQ 4, water 1 | Spray coating | 125 | 1150–1250 °C, 2 h in air; then 1350–1500 °C, 4 h in Ar | 2.31 μm (MF) | ||
| Membranes | Precursor | Supports | Deposition Temperature | Ref. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiCN | SiH4/C2H2/NH3 | α-Al2O3; disk | 1050 °C in Ar | [37][60] |
|
[25][48] | ||||||
| Flat tube, 1.8 μm | ||||||||||||
| SiCO | na. | SiH2Cl2/C2H2/H2SiC powder (0.55 μm), IPA 1, PVA 3, PEG 3, Darvan-CN 2, water | γ-Al21 | ODip-coating | 12–30 | 3900–1300 °C, 1 h | 75–155 nm (MF) | /α-Al2O3; tube
|
[ | 800–900 °C in H2 | [38][39][61,26][49] | |
| 62 | ] | Flat tube, 34.92 μm | na. | SiC powder (22 µm), B4C 5, PVA 3, TMAOH 2, water | Dip-coating | ~100 | 2200–2250 °C | 9.93 μm (MF) | ||||
| SiC | Triisopropylsilane (TPS) | SiC; disk; and tube | 760–800 °C in Ar/He | [36][59] |
|
[27][50] | ||||||
| Tube, 15 μm | na. | α-SiC powder (0.4 μm and 0.6 μm), Al(NO3)3·9H2O 5, Optapix CS-76 3, polysaccharide dicarbonic acid polymer 3, water 1 | ||||||||||
| SiC | Dip-coating | 27.3–29.4 | 1600–1900 °C | 0.35 μm (MF) | Silacyclobutane (SCB) | Ni-γ-Al2O3/α-Al2O3; tube | 515 °C in Ar | [40][63] |
|
[28][51] | ||
| Flat sheet, 5.6–14.1 μm | na. | SiC powders (0.5 µm and 3 µm), PAA 2, CMC 3, water 1 | Dip-coating | 60 | 1900–2000 °C in vacuum | 0.5 μm (1900 °C); 1.4 μm (2000 °C) (MF) |
Dip-coating | 10–19 | 200 °C, 1 h; 400 °C, 1 h; and then 750 °C, 2 h | <50 nm (UF) |
|
[30][53] |
| Tube/na. | SiC powder (0.6 µm), acetone; pore diameter =130 nm | PS 7, toluene 1, AHPCS 6, hexane 1 | Slip-casting + dip-coating |
7 | 200 °C, 1 h, 400 °C, 1 h, and then 750 °C, 2 h in Ar; 450 °C, 2 h in air | Nanoporous SiC membranes |
|
[31][54] |
2.1. Chemical Vapor Deposition/Infiltration Route
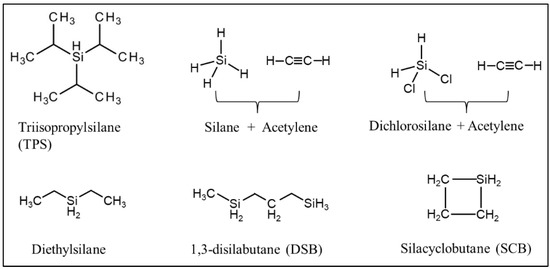
CVD/CVI involves exposing a heated substrate to one or more volatile precursors (e.g., organosilica) that react or decompose on the substrate surface (or on the pore walls) to deposit the desired thin film [33][34][56,57]. CVD/CVI is a versatile process for manufacturing coatings/films, fibers, powders, and monolithic components [35][58]. During the formation of SiC membranes, a schematic diagram of the phenomena that could occur via the CVD/CVI deposition process is shown in Figure 23. The precursor (e.g., triisopropylsilane, TPS) is carried by the carrier gas (e.g., Ar) into the pore space of the support, where it reacts to form SiC on the pore surface. SiC gradually shrinks/plugs the pores (especially the macropores) and forms a dense membrane layer [36][59]. Table 23 lists the representative processing parameters of the SiC membranes obtained by CVD/CVI techniques using the typical precursors shown in Figure 34. It is worth noting that the vast majority of support materials used to fabricate inorganic membranes, as well as SiC membranes, are α-Al2O3. This is due mainly to the ease of commercialization and high economic competitiveness of α-Al2O3.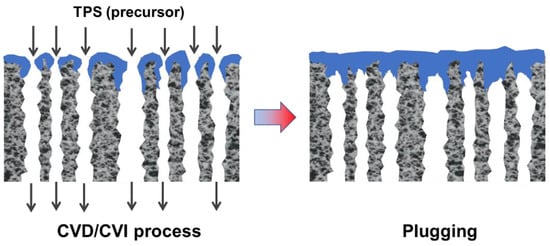
| SiC | ||||||||
| 1,3-disilabutane (DSB); TPS | ||||||||
| α-Al | 2 | O3; γ-Al2O3/α-Al2O3; tube | TPS: 700–800 °C in He, and annealed at 1000 °C; DSB: 650–750 °C in He |
[41][42][64,65 |
|
[29][52] | ||
| ] | Flat disk, <100 μm | Consisting of several SiC layers; pore diameter <300 nm |
α-SiC powder (0.4 µm), AHPCS 6, hexane 1, hexane/tetradecane 1 |
2.2. Pyrolysis of the Polymeric Precursor Route
Another well-known method for preparing SiC membranes is the pyrolysis of coated polymeric precursors. The greatest advantage of this method over the CVD/CVI technique is its processing simplicity, low thermal treatment (pyrolysis) temperatures (≤850 °C), and the fact that production can be carried out either continuously or in a batch process. Furthermore, the preceramic polymeric precursor approach features significant advantages in manufacturing membranes with controlled properties and structures, such as composition, hydrophilic/hydrophobic properties, and pore size, which ultimately affect SiC membrane performance. Figure 56 presents a general schematic of the pyrolysis of the preceramic precursor for manufacturing SiC membranes. In particular, the employed polymeric precursors generally undergo three steps using this route to form SiC membranes, depending on the temperature (these steps are shown at the bottom of Figure 56): coating/shaping, curing for cross-linking, and pyrolysis under an inert atmosphere to complete the polymer-to-ceramic conversion [43][66]. In this multi-step process, each step is important for obtaining high-quality membranes.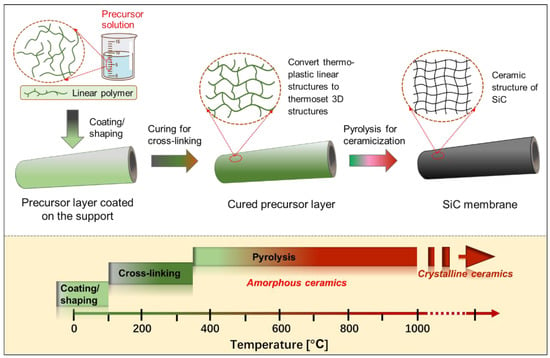
2.2.1. Si-Containing Precursors
The fabrication of SiC ceramics by the pyrolysis of a polymeric precursor process is strongly influenced by the chemistry and architecture of the Si-based preceramic precursors, their processing routes, and the parameters used for their pyrolysis. To be suitable for producing SiC ceramic materials, Si-based polymers must meet the following important requirements: (i) the polymers should have a sufficiently high molecular weight to avoid volatilization of components with low molecular weights during subsequent curing and pyrolysis processes; (ii) they should have appropriate rheological characteristics, as well as solubility for the coating/shaping process; (iii) they should have latent reactivity, provided by the presence of specific functional groups (e.g., Si–H, N–H, Si–OH, and Si–CH=CH2), which induce cross-linking during the curing process upon exposure to thermal stimuli, chemical stimuli, or irradiation (e.g., UV, electro-beam, and γ-ray).
2.2.2. Coating/Shaping
The quality of the coating/shaping depends on the deposition techniques and the solution properties of the precursors. Precursor coating methods mainly include dip-coating [44][31], wipe-coating [32][55], casting [31][54], spin-coating [45][80], spray-coating [25][48], and even three-dimensional (3D) printing [46][81]. Dip-coating and wipe-coating are the most frequently used methods for fabricating membranes because of their relative simplicity. An important factor affecting the coating layers is the viscosity (a rheological characteristic) of the coating solutions, which should be controlled carefully by changing the molecular architectures of the precursors, concentration, and solvent species to achieve coating layers with high quality. This is because the viscosity of the coating solutions could affect the thickness and uniformity of the membrane layers.
2.2.3. Curing and Pyrolysis Processes
Curing for Cross-Linking
The coated precursor typically requires to be cured at low temperatures (up to 300–400 °C) prior to pyrolysis [47][48][71,83], which plays an important role in determining the final quality of SiC ceramic materials, including their microstructural properties. The curing process of polymeric precursors for cross-linking converts the thermoplastic polymers into thermosetting polymers via a series of reactions, such as dehydrogenation and oxidation, which prevents the coating layers/shapes from fusing together during pyrolysis [49][84]. Additionally, curing is known to promote high ceramic yields of polymeric precursors because cross-linking prevents the volatilization of precursor components with low molecular weight at high temperatures. To date, several curing techniques have been carried out to produce SiC membranes, such as ultraviolet (UV) radiation, electron beam (EB)/γ-ray irradiation [50][51][85,86], and conventional thermal treatment under an oxidizing or inert atmosphere [52][53][73,87]. These curing techniques induce various condensation (dehydrogenation or demethanization) and addition reactions converting linear polymer networks to 3D polymer networks.
Pyrolysis for Polymer-To-Ceramic Conversion
After curing for cross-linking, further thermal treatment at elevated temperatures (usually ≥300 °C) and under an inert atmosphere, i.e., pyrolysis, results in an organic-to-inorganic conversion (from thermoset polymers to amorphous SiC ceramics) [43][52][66,73]. This conversion is caused mainly by radicals, condensation (dehydrogenation and demethanization), and rearrangement reactions, which lead to the cleavage of chemical bonds and the formation of new bonds accompanied by the elimination of organic groups and the release of gases, such as H2, CH4, and C6H6 [32][55]. For most polymeric precursors, the conversion from polymeric precursor to amorphous ceramic is complete at <900 °C, followed by crystallization from the amorphous phase at higher temperatures (>1100 °C) and resulting in phase separation [32][47][52][54][55][56][34,55,71,72,73,82].
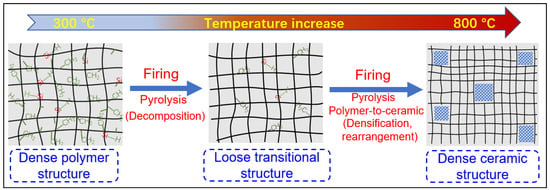
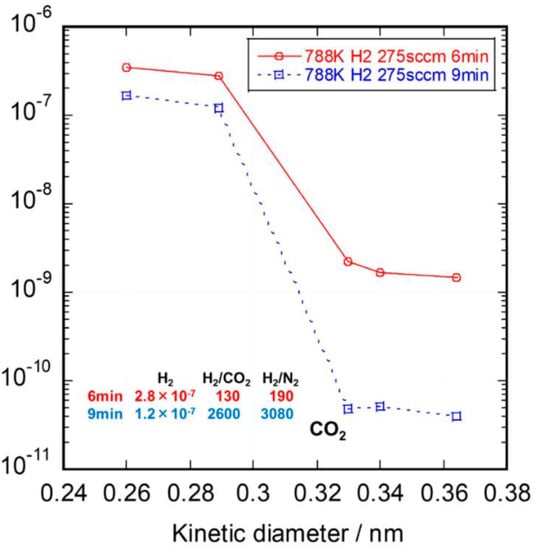
During the organic-to-inorganic ceramic transformation, the microstructure of polymeric precursors undergoes dramatic changes with increasing pyrolysis temperature [32][55]. As shown in Figure 67, the microporous properties and pore structure parameters (BET and micropore volume) of the pyrolytic precursor (polytitanocarbosilane, TiPCS) powders were analyzed by N2 adsorption–desorption isotherms [32][55]. The adsorption capacity, micropore volume, and BET surface area increased by firing from 500 °C to 650 °C and then decreased with increasing pyrolysis temperature from 650 °C to 1000 °C. It is worth noting that this is a general conclusion because PCS [55][57][72,96], polydimethylsilane (PMS) [58][97], and AHPCS [52][73] precursors follow the same trends within a similar pyrolysis temperature range of 300–850 °C. These trends suggest that the decomposition of organic groups generates many micropores at moderate temperatures and the micropores are then narrowed gradually as the pyrolysis temperature increases because of densification and rearrangement reactions.
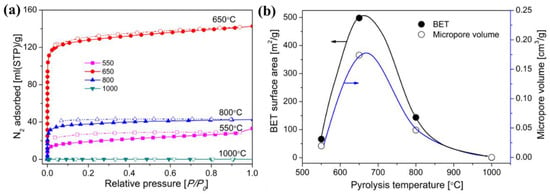
Figure 68. (a) N2 adsorption–desorption isotherms at 77 K, (b) BET surface area and micropore volume (at a relative pressure of P/P0 = 0.01, pore size ≤ 1 nm) of pyrolyzed precursor (TiPCS) powders. Adapted from [32][55] with permission from Elsevier.