Osteoporosis is a highly prevalent bone disorder especially in post-menopausal women. Lifestyle interventions such as physical activity and nutrition play an important role in the prevention and treatment of bone mineral loss. Phytate (myo-inositol hexakisphosphate or InsP6) is the main phosphorus reservoir that is present in almost all wholegrains, legumes, and oilseeds. It is a major component of the Mediterranean and Dietary Approaches to Stop Hypertension (DASH) diets. Phytate is recognized as a nutraceutical and is classified by the Food and Drug Administration (FDA) as Generally Recognized As Safe (GRAS). Phytate has been shown to be effective in treating or preventing certain diseases. Phytate has been shown to inhibit calcium salt crystallization and, therefore, to reduce vascular calcifications, calcium renal calculi and soft tissue calcifications.
1. Introduction
The influence of nutrition is crucial for the prevention and even the cure of several diseases. In fact, healthy eating patterns and regular physical activity could prevent up to 80% of instances of coronary heart disease, 90% of type 2 diabetes (T2DM) cases and 30% of all cancers
[1]. The Mediterranean diet is characterized by a high intake of plant food (e.g., fruits, vegetables, breads and cereals), beans, nuts, seeds and olive oil
[2]. The traditional Mediterranean diet assumes the consumption of about 1 g of phytate per day, accompanied by an appropriate amount of other minerals and bioactive components
[3]. Other diets, such as the European/American diet, can provide up to 2 g of phytate per day
[4]. The administration of high doses of phytate must be accompanied by an adequate content of minerals. The Mediterranean and D
ietary A
pproaches to Stop Hypertension (DASH) SH diet provide an adequate amount of legumes, whole cereals and nuts, which are rich in phytate and other minerals, in order to not negatively affect the mineral balance
[3][4][3,4]. In this way, Serra-Majem et al., 2009
[5], showed that a higher adherence to a Mediterranean diet was associated with a lower inadequate intake of zinc, iodine, vitamin E, magnesium, iron, vitamin B1, vitamin A, selenium, vitamin C and folic acid in comparison with other Western diets such as the American diet.
Myo-inositol hexakisphosphate (InsP6), also known as phytic acid or phytate, is the main phosphorus reservoir that is present in almost all wholegrains, legumes and oilseeds
[4]. In plant foods, it is found as a calcium–magnesium salt, called phytin. When phytate is consumed in large amounts, by itself and without being processed/cooked, it can reduce the absorption of some minerals. This has led to phytate being classified by some authors as an antinutrient. Nevertheless, this effect is only seen in laboratory conditions, and real-world data in humans do not demonstrate mineral deficiencies induced by phytate intake. Phytate is instead considered a nutraceutical: a compound that could treat or prevent disease or disorders through a variety of bioactive (e.g., antioxidant, immunomodulatory, lipid lowering) functions
[1]. These properties and multiple health benefits have been repeatedly seen in the scientific literature. The F
ood and D
rug Administration (FDA)A classify phytin as Generally Recognized As Safe (GRAS)
[4].
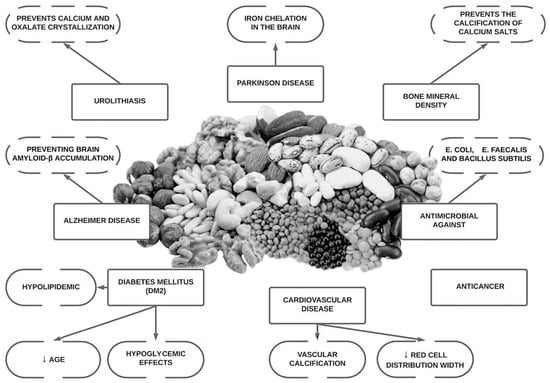
Since the discovery of the molecule phytate, research has shown that it may aid essential physiological functions, as well as offer anti-oxidant, anti-inflammatory, anti-cancer properties, be anti-diabetic, neuroprotective, antimicrobial and moreover, have the ability to prevent bone mass loss and decrease pathological calcification such as vascular calcification and renal lithiasis
[4][6][7][8][4,6,7,8]. It has also demonstrated that the ingestion of phytate also generate other inositol phosphates (InsP5, InsP4, InsP3, InsP2), which can also play an important role in these pathological conditions
[8]. Phytate has shown promising results in the treatment and prevention of medical conditions that have no current treatment (or where treatment options are prohibitively expensive) in in vitro data, animal models and in some human clinical trials (
Figure 1).
Figure 1.
Overview of potential health benefits of phytate.
2. Background and In Vitro Studies
Phytate is an inhibitor of crystallization due to the capacity of binding to the crystal surface. The adsorption of phytate to the crystal faces can inhibit pathological calcifications in vivo and in vitro as mentioned, but can also inhibit hydroxyapatite dissolution and bone resorption
[9]. It is important to remark that pyrophosphate and bisphosphonates were discovered previously as inhibitors of both crystal formation and dissolution by binding strongly to HAP crystals. In fact, the bisphosphonates are widely use as drugs for bone mass loss disease
[10][54].
Phytate has shown the ability in vitro to reduce the osteoclastogenesis of human primary osteoclasts (human peripheral blood mononuclear cell culture (PBMNC) and mouse macrophage RAW264.7 cell lines)
[11][55]. Similar results have been obtained in previous in vitro studies
[12][13][14][15][56,57,58,59]. In a recent in vitro study
[9], phytate was able to inhibit acid-driven HAP dissolution. Moreover, the inhibitory effect of phytate on HAP dissolution was greater than that exhibited by etidronate and similar to that of alendronate
[9]. Additionally, phytate inhibited HAP dissolution in a concentration-dependent manner
[9]. Phytate could help in two opposite processes: to prevent calcification and to decrease bone mass loss
[9].
3. Animal Studies
In an animal model study on postmenopausal osteoporosis carried out over 12 weeks on ovariectomized rats, one group was fed with AIN-76A (no phytate diet) and the other group with AIN-76 enriched with 1% phytin. The phytin-consuming group showed an increase in bone mineral density in femoral bones and L4 vertebrae. Phytin reduced the bone density loss caused by estrogen deficiency
[16][60].
On the other hand, Kim et al., 2020
[17][40], challenged the previous results. In a study using four-week-old male and female Sprague Dawley rats the systemic effects of dietary phytate were evaluated. Rats were fed AIN-93G diets supplemented with 0%, 1%, 3%, or 5% phytate over 12 weeks with a constant supplementation of calcium concentration. The trial showed that in the AIN-93G diet plus phytate supplementation caused a time and concentration dependent impairment of the renal reabsorption of calcium and phosphate accompanied by PTH increase, predisposing the rats to the development of hypophosphatemic rickets. Once again, it is necessary to consider that these authors used sodium phytate in their study, and not the calcium–magnesium salt, which is the form in which it is found in food.
4. Epidemiological Studies
The high number of epidemiological studies linking phytate consumption with an improvement of bone health is worth of mention. A descriptive cross-sectional pilot study
[18][61] carried out among 143 postmenopausal women examined the relationship between urinary phytate concentration and risk of fracture within 10 years (using the FRAX model). The risk of major osteoporotic fracture and hip fracture were higher in women with low urinary phytate levels. This difference was higher in women with at least one risk factor for osteoporosis. Similar results were obtained previously by Lopez-González et al., 2013
[19][62], in a study carried out among 157 postmenopausal women; low InsP6 levels were associated with significantly greater bone mass loss in the lumbar spine and the 10-year fracture probability (calculated by the FRAX model) was also significantly higher in the low-phytate group compared with the high-phytate group, both in hip and major osteoporotic fracture
[19][62]. Moreover, in a prospective study with 1473 subjects they found that the higher the phytate consumption, the greater mineral density
[20][63]. Similar results with a similar design study were found by Lopez-González et al., 2011
[21][64], where phytate consumption was measured by food questionnaires and bone mineral density was evaluated by dual-X-ray double-energy absorptiometry. The results indicated that adequate phytate consumption may play a significant role in the prevention of bone mineral density loss in postmenopausal women
[21][64].
Sanchis et al., 2021
[9], carried out a cross-sectional study in which 415 women completed a validated 14-item questionnaire designed to estimate adherence to the Mediterranean Diet and phytate consumption and where bone mineral density of the L1-L4 was evaluated by dual energy X-ray absorptiometry (DXA). The results showed a significant association between low phytate consumption and low bone mineral density at lumbar spine
[9]. According to these data, the ingestion of at least 307 mg/day would prevent bone mineral loss. In a practical sense, this would be highly achievable since the Mediterranean diet is associated with a phytate intake of 1–2 g per day
[22][23][31,65].
In this sense, the Mediterranean diet has been demonstrated to be an effective method to increase bone mineral density. As mentioned before, diet is a modifiable factor which is crucial to reduce the risk of osteoporosis
[24][66]. The Mediterranean diet, along with other diets such as DASH, is rich in fruits, vegetables, legumes and nuts, all of which are associated with better bone health in epidemiological studies
[24][25][26][27][52,66,67,68].
5. Clinical Trials in Humans
As mentioned previously, Guimerà et al., 2022
[28][49] conducted the first controlled randomized trial on the effects in humans of daily phyate supplementation on bone mineral density (BMD) as a secondary outcome. They used ß-Crosslaps as a serum maker for predicting BMD and the response to antiresorptive treatment. Patients with hypercalciuria (>250 mg/24 h) and osteopenia or osteoporosis (determined by densitometry) in the femur and/or spine who received daily administration of 380 mg capsule of calcium–magnesium InsP6 had significantly lower levels of ß-Crosslaps in comparation with the placebo group after three months of supplementation.